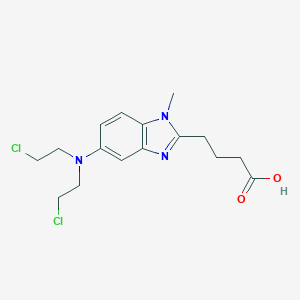
Bendamustine
概要
説明
ベンダムスチンは、化学療法薬であり、主に慢性リンパ性白血病、多発性骨髄腫、および非ホジキンリンパ腫の治療に使用されます . これは、アルキル化剤のクラスに属し、DNAとRNAの機能を阻害することにより細胞死を引き起こします . ベンダムスチンは、1960年代初頭にドイツ民主共和国で初めて合成され、2008年に米国で医療用として承認されました .
2. 製法
合成経路および反応条件: ベンダムスチン塩酸塩は、複数段階のプロセスによって合成できます。 重要な中間体である1-メチル-5-[ビス(2-クロロエチル)アミノ]-1H-ベンゾイミダゾール-2-イル]リチウムブタノエートが調製され、その後ベンダムスチン塩酸塩に変換されます . このプロセスには、トリフルオロ酢酸やアセトニトリルなどのさまざまな試薬や溶媒が使用されます .
工業生産方法: ベンダムスチン塩酸塩の工業生産は、高純度(≥99%)を目指しており、精製には高速液体クロマトグラフィー(HPLC)などの手順が含まれます . このプロセスは、経済的で環境に優しい設計になっており、有害な化学物質の使用を避けています .
準備方法
Synthetic Routes and Reaction Conditions: Bendamustine hydrochloride can be synthesized through a multi-step process. The key intermediate, 1-methyl-5-[bis(2-chloroethyl)amino]-1H-benzimidazol-2-yl]lithium butanoate, is prepared and then converted to this compound hydrochloride . The process involves the use of various reagents and solvents, including trifluoroacetic acid and acetonitrile .
Industrial Production Methods: The industrial production of this compound hydrochloride aims for high purity (≥99%) and involves steps such as high-performance liquid chromatography (HPLC) for purification . The process is designed to be economical and environmentally friendly, avoiding the use of hazardous chemicals .
化学反応の分析
反応の種類: ベンダムスチンは、アルキル化、加水分解、酸化など、いくつかの種類の化学反応を起こします . アルキル化剤として、DNA塩基間に鎖内および鎖間架橋を形成し、細胞死につながります .
一般的な試薬と条件:
アルキル化: ベンダムスチンは、DNA塩基に共有結合する求電子性アルキル基を形成します.
加水分解: この化合物は、肝臓で不活性な代謝産物に加水分解されます.
酸化: ガンマヒドロキシベンダムスチンやN-デスメチルベンダムスチンなどのマイナーな代謝産物は、酸化によって形成されます.
主な生成物: これらの反応から形成される主な生成物には、不活性な代謝産物と架橋されたDNAが含まれます .
4. 科学研究への応用
ベンダムスチンは、幅広い科学研究の応用を持っています:
科学的研究の応用
Bendamustine has a wide range of scientific research applications:
Chemistry: It is studied for its unique bifunctional properties, combining alkylating and antimetabolite activities.
Biology: Research focuses on its effects on DNA and RNA, as well as its immunomodulatory properties.
Industry: It is used in the development of new chemotherapy regimens and combination therapies.
作用機序
ベンダムスチンは、複数のメカニズムを介してその効果を発揮します:
アルキル化: DNA塩基間に鎖内および鎖間架橋を形成し、細胞死につながります.
DNA損傷応答の活性化: ベンダムスチンは、DNA損傷ストレス応答とアポトーシスを活性化します.
有糸分裂チェックポイントの阻害: 有糸分裂チェックポイントを阻害し、有糸分裂カタストロフィーを誘発します.
6. 類似の化合物との比較
ベンダムスチンは、アルキル化剤とプリンアナログの両方の特性を組み合わせた、2機能性であることが特徴です . 類似の化合物には以下が含まれます:
シクロホスファミド: 化学療法で使用される別のアルキル化剤です。
メルファラン: 多発性骨髄腫に使用されるアルキル化剤です。
クロラムブシル: 慢性リンパ性白血病に使用されます。
これらの化合物と比較して、ベンダムスチンは、静止期と分裂期の両方の細胞に対して、安定性と活性の幅広さが向上したことが示されています .
類似化合物との比較
Bendamustine is unique due to its bifunctional nature, combining properties of both alkylating agents and purine analogs . Similar compounds include:
Cyclophosphamide: Another alkylating agent used in chemotherapy.
Melphalan: An alkylating agent used for multiple myeloma.
Chlorambucil: Used for chronic lymphocytic leukemia.
Compared to these compounds, this compound has shown increased stability and a broader range of activity against both quiescent and dividing cells .
特性
IUPAC Name |
4-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H21Cl2N3O2/c1-20-14-6-5-12(21(9-7-17)10-8-18)11-13(14)19-15(20)3-2-4-16(22)23/h5-6,11H,2-4,7-10H2,1H3,(H,22,23) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
YTKUWDBFDASYHO-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN1C2=C(C=C(C=C2)N(CCCl)CCCl)N=C1CCCC(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H21Cl2N3O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
3543-75-7 (hydrochloride) | |
| Record name | Bendamustine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0016506277 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID2046888 | |
| Record name | Bendamustine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2046888 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
358.3 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Bendamustine is a bifunctional mechlorethamine derivative capable of forming electrophilic alkyl groups that covalently bond to other molecules. Through this function as an alkylating agent, bendamustine causes intra- and inter-strand crosslinks between DNA bases resulting in cell death. It is active against both active and quiescent cells, although the exact mechanism of action is unknown., Multiple myeloma is a fatal hematological disease caused by malignant transformation of plasma cells. Bendamustine has been proven to be a potent alternative to melphalan in phase 3 studies, yet its molecular mode of action is still poorly understood. The four-myeloma cell lines NCI-H929, OPM-2, RPMI-8226, and U266 were cultured in vitro. Apoptosis was measured by flow cytometry after annexin V FITC and propidium iodide staining. Cell cycle distribution of cells was determined by DNA staining with propidium iodide. Intracellular levels of (phosphorylated) proteins were determined by western blot. /It was shown/ that bendamustine induces apoptosis with an IC50 of 35-65 mug/ml and with cleavage of caspase 3. Incubation with 10-30 mug/ml results in G2 cell cycle arrest in all four-cell lines. The primary DNA-damage signaling kinases ATM and Chk2, but not ATR and Chk1, are activated. The Chk2 substrate Cdc25A phosphatase is degraded and Cdc2 is inhibited by inhibitory phosphorylation of Tyr15 accompanied by increased cyclin B levels. Additionally, p53 activation occurs as phosphorylation of Ser15, the phosphorylation site for ATM. p53 promotes Cdc2 inhibition by upregulation of p21. Targeting of p38 MAPK by the selective inhibitor SB202190 significantly increases bendamustine induced apoptosis. Additionally, SB202190 completely abrogates G2 cell cycle arrest. Bendamustine induces ATM-Chk2-Cdc2-mediated G2 arrest and p53 mediated apoptosis. Inhibition of p38 MAPK augments apoptosis and abrogates G2 arrest and can be considered as a new therapeutic strategy in combination with bendamustine., Microarray-based gene expression profiling, real-time PCR, immunoblot, cell cycle, and functional DNA damage repair analyses were used to characterize response to bendamustine and compare it with chlorambucil and phosphoramide mustard. Bendamustine displays a distinct pattern of activity unrelated to other DNA-alkylating agents. Its mechanisms of action include activation of DNA-damage stress response and apoptosis, inhibition of mitotic checkpoints, and induction of mitotic catastrophe. In addition, unlike other alkylators, bendamustine activates a base excision DNA repair pathway rather than an alkyltransferase DNA repair mechanism. These results suggest that bendamustine possesses mechanistic features that differentiate it from other alkylating agents and may contribute to its distinct clinical efficacy profile., Bendamustine is a bifunctional mechlorethamine derivative containing a purine-like benzimidazole ring. Mechlorethamine and its derivatives form electrophilic alkyl groups. These groups form covalent bonds with electron-rich nucleophilic moieties, resulting in interstrand DNA crosslinks. The bifunctional covalent linkage can lead to cell death via several pathways. Bendamustine is active against both quiescent and dividing cells. The exact mechanism of action of bendamustine remains unknown. | |
| Record name | Bendamustine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06769 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bendamustine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7763 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
16506-27-7 | |
| Record name | Bendamustine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=16506-27-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Bendamustine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0016506277 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bendamustine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06769 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bendamustine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID2046888 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 16506-27-7 | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | BENDAMUSTINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/9266D9P3PQ | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Bendamustine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7763 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。


![1,4-Dimethylbenzo[a]pyrene](/img/structure/B91590.png)
