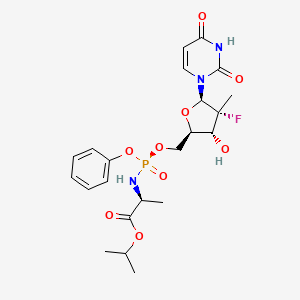
Sofosbuvir
- 専門家チームからの見積もりを受け取るには、QUICK INQUIRYをクリックしてください。
- 品質商品を競争力のある価格で提供し、研究に集中できます。
説明
ソフォスブビルは、慢性C型肝炎ウイルス(HCV)感染症の治療に使用される直接作用型抗ウイルス薬です。 ヌクレオチドアナログファミリーの薬剤に属し、ウイルス複製に必須なHCV NS5B RNA依存性RNAポリメラーゼを阻害することで作用します . ソフォスブビルは2007年に発見され、2013年に米国で医療用として承認されました . ソバルディという商品名で販売されており、世界保健機関の必須医薬品リストに掲載されています .
作用機序
ソフォスブビルは、体内では活性型であるGS-461203に代謝されるプロドラッグです . この活性型は、欠損基質として作用することにより、HCV NS5B RNA依存性RNAポリメラーゼを阻害し、それによりウイルスRNA鎖を終了させて複製を阻害します . この酵素の阻害は、HCV複製を抑制し、持続的なウイルス学的反応を達成するために不可欠です .
類似の化合物との比較
ソフォスブビルは、次のような他の直接作用型抗ウイルス薬としばしば比較されます。
レディパスビル: HCVの治療に、ソフォスブビルと組み合わせて使用されます.
ベルパスビル: HCVのパンジェノタイプ治療に、ソフォスブビルと組み合わせて使用される別の抗ウイルス薬です.
ダクラタスビル: HCVの治療に、ソフォスブビルと組み合わせて使用されます.
シメプレビル: HCVの治療に、ソフォスブビルと組み合わせて使用されます.
ソフォスブビルは、耐性発生に対する高い障壁と、複数のHCV遺伝子型にわたる有効性で特徴付けられます . 他の抗ウイルス薬との組み合わせは、その有効性を高め、さまざまなHCV遺伝子型の治療における応用範囲を拡大します .
準備方法
ソフォスブビルの合成には、重要な中間体の調製と最終的なカップリング反応を含むいくつかのステップが関与します。合成経路の1つは、(2’R)-2’-デオキシ-2’-フルオロ-2’-メチルウリジンを出発原料として使用します。 この化合物は、ルイス酸とアルカリの存在下、有機溶媒中で他の原料と反応させてソフォスブビルを生成します . 反応条件は穏和であり、このプロセスは大規模な工業生産に適しています .
別の方法は、より立体選択的な調製を伴い、中間体を分離する必要なく、目的の異性体を生成することができます。 この方法は、生産コストを削減し、工業化された生産にも適しています .
化学反応の分析
ソフォスブビルは、次のようなさまざまな化学反応を起こします。
酸化: ソフォスブビルは酸化されて、その活性代謝物であるGS-461203(トリリン酸)を形成することができます.
還元: 還元反応は、ソフォスブビルとは一般的に関連していません。
これらの反応に使用される一般的な試薬と条件には、有機溶媒、ルイス酸、アルカリなどがあります . これらの反応から生成される主な生成物には、ソフォスブビルの活性なトリリン酸形態と、その合成に使用される他の中間体が含まれます .
科学研究への応用
ソフォスブビルは、次のようないくつかの科学研究への応用があります。
化学: ソフォスブビルは、ヌクレオチドアナログとその合成に関する研究においてモデル化合物として使用されます.
生物学: RNAウイルスの複製メカニズムと、ウイルスポリメラーゼの阻害を研究するために使用されます.
科学的研究の応用
Sofosbuvir has several scientific research applications, including:
Chemistry: This compound is used as a model compound in the study of nucleotide analogs and their synthesis.
Medicine: this compound is primarily used in the treatment of chronic hepatitis C infections.
Industry: This compound’s synthesis and production methods are studied to improve industrial-scale manufacturing processes and reduce production costs
類似化合物との比較
Sofosbuvir is often compared with other direct-acting antiviral agents, such as:
Ledipasvir: Used in combination with this compound for the treatment of HCV.
Velpatasvir: Another antiviral agent used in combination with this compound for pan-genotypic treatment of HCV.
Daclatasvir: Used in combination with this compound for the treatment of HCV.
Simeprevir: Used in combination with this compound for the treatment of HCV.
Remdesivir: An antiviral agent used for the treatment of COVID-19, which also targets RNA-dependent RNA polymerase.
This compound is unique in its high barrier to resistance development and its effectiveness across multiple HCV genotypes . Its combination with other antiviral agents enhances its efficacy and broadens its application in treating various HCV genotypes .
特性
| Sofosbuvir is a direct-acting antiviral agent (pan-genotypic polymerase inhibitor) against the hepatitis C virus. HCV RNA replication is mediated by a membrane-associated multiprotein replication complex. The HCV polymerase (NS5B protein) is an RNA-dependent RNA polymerase (RdRp). It is the essential initiating and catalytic subunit of this replication complex and is critical for the viral replication cycle. There is no human homolog for HCV NS5B RdRp. Sofosbuvir is a monophosphorylated pyrimidine nucleotide prodrug that undergoes intracellular metabolism to form the pharmacologically active uridine analog triphosphate (GS-461203). GS-461203 competes with natural nucleotides for incorporation (by HCV NS5B) into the nascent RNA strand during replication of the viral genome. GS-461203 differs from endogenous pyrimidine nucleotides in that it has been modified at the 2' position with the addition of a methyl and a fluoro functional group. Incorporation of GS-461203 into nascent RNA strongly reduces the efficiency of further RNA elongation by RdRp, resulting in premature termination of RNA synthesis. The stopping of viral replication leads to a rapid decline of HCV viral load and clearing of HCV levels in the body. | |
CAS番号 |
1190307-88-0 |
分子式 |
C22H29FN3O9P |
分子量 |
529.5 g/mol |
IUPAC名 |
propan-2-yl 2-[[[(4R)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyloxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate |
InChI |
InChI=1S/C22H29FN3O9P/c1-13(2)33-19(29)14(3)25-36(31,35-15-8-6-5-7-9-15)32-12-16-18(28)22(4,23)20(34-16)26-11-10-17(27)24-21(26)30/h5-11,13-14,16,18,20,28H,12H2,1-4H3,(H,25,31)(H,24,27,30)/t14?,16?,18?,20?,22-,36+/m1/s1 |
InChIキー |
TTZHDVOVKQGIBA-IECBXEDQSA-N |
SMILES |
CC(C)OC(=O)C(C)NP(=O)(OCC1C(C(C(O1)N2C=CC(=O)NC2=O)(C)F)O)OC3=CC=CC=C3 |
異性体SMILES |
CC(C)OC(=O)C(C)N[P@](=O)(OCC1C([C@@](C(O1)N2C=CC(=O)NC2=O)(C)F)O)OC3=CC=CC=C3 |
正規SMILES |
CC(C)OC(=O)C(C)NP(=O)(OCC1C(C(C(O1)N2C=CC(=O)NC2=O)(C)F)O)OC3=CC=CC=C3 |
外観 |
white solid powder |
Color/Form |
White to off-white crystalline solid |
純度 |
>98% (or refer to the Certificate of Analysis) |
賞味期限 |
>2 years if stored properly |
溶解性 |
Slightly soluble in water |
保存方法 |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
同義語 |
PSI-7977; PSI 7977; PSI7977; GS7977; GS-7977; GS 7977; Sofosbuvir; Sovaldi; Virunon; Vosevi; Hepcinat; Hepcvir; Resof; |
製品の起源 |
United States |
Q1: What is the mechanism of action of Sofosbuvir?
A1: this compound is a pan-genotypic inhibitor of the Hepatitis C Virus (HCV) NS5B RNA polymerase, a key enzyme responsible for viral replication. [, , , , , ] It acts as a chain-terminating nucleotide analogue, interfering directly with the HCV life cycle by suppressing viral replication. [, , ]
Q2: How does this compound affect HCV after binding to NS5B polymerase?
A2: Upon entering hepatocytes (liver cells), this compound is metabolized into its active form, a nucleoside triphosphate. This active metabolite competes with naturally occurring nucleotides, ultimately leading to the termination of RNA chain elongation during HCV replication. [, ]
Q3: What is the molecular formula and weight of this compound?
A3: The scientific literature provided does not specify the exact molecular formula and weight of this compound.
Q4: How stable is this compound under various storage conditions?
A5: While specific stability data is limited in the provided research, this compound has been successfully formulated into tablets for oral administration. [, , , , , ] Stability indicating HPTLC methods have been developed for this compound and Daclatasvir in combination, highlighting the need for validated methods to assess drug stability. []
Q5: Does this compound have any catalytic properties?
A5: this compound itself doesn't possess catalytic properties. Its antiviral action stems from inhibiting the catalytic activity of the HCV NS5B polymerase.
Q6: Have any computational studies been conducted on this compound?
A6: There is no mention of specific computational chemistry studies on this compound within the provided research articles.
Q7: What formulation strategies have been explored for this compound?
A9: this compound is primarily formulated as tablets for oral administration. [, , , , , ] Further research exploring strategies to optimize this compound formulation for improved stability, solubility, and bioavailability is warranted.
Q8: Is there information regarding this compound's SHE compliance?
A8: The provided scientific research focuses primarily on the clinical efficacy and safety of this compound. Information regarding specific SHE (Safety, Health, and Environment) regulations and compliance is not discussed.
Q9: What is known about the pharmacokinetic profile of this compound?
A11: this compound is readily absorbed after oral administration, achieving peak plasma concentrations in approximately 30 minutes. [, ] It undergoes extensive hepatic metabolism, primarily by phosphorylation, into its active triphosphate form. [, , ] The primary metabolite, GS-331007, is largely inactive against HCV and is eliminated in the urine. []
Q10: What cell-based assays have been used to evaluate this compound's activity?
A13: this compound's antiviral activity has been extensively studied in cell culture using HCV replicons. [, , ] These replicons are engineered systems that allow for the replication of the HCV genome in cultured cells, enabling the assessment of antiviral activity. Resistance selection experiments have also been conducted in cell culture to understand the emergence of resistance to this compound. []
Q11: What is the efficacy of this compound in clinical trials?
A14: Clinical trials have demonstrated that this compound-based regimens achieve high sustained virological response (SVR) rates, signifying viral eradication, across various HCV genotypes and patient populations. [, , , , , , , , , , , , , , ] Notably, this compound-containing regimens have shown efficacy in treatment-naïve and treatment-experienced patients, as well as in those with cirrhosis. [, , , , , , , , , , , , , , ]
Q12: What are the primary mechanisms of resistance to this compound?
A15: The primary mechanism of this compound resistance involves mutations in the NS5B polymerase, specifically the S282T substitution. This mutation has been identified across all HCV genotypes and confers reduced susceptibility to this compound. [, , ]
Q13: Does cross-resistance occur between this compound and other antivirals?
A16: While S282T is the primary resistance mutation, other substitutions in NS5B can emerge during this compound treatment, and their impact on resistance and cross-resistance with other antivirals requires further investigation. [] Clinical studies have shown that this compound-containing regimens, particularly this compound/Velpatasvir/Voxilaprevir, are effective as salvage therapies in patients who have failed prior DAA treatments. []
Q14: Are there any specific drug delivery strategies for this compound?
A17: The provided research primarily focuses on the oral formulation and efficacy of this compound. [, , , , , ] Specific drug delivery and targeting strategies for this compound are not discussed in detail within these articles.
Q15: Are there any biomarkers associated with this compound treatment response?
A18: The research primarily focuses on sustained virologic response (SVR) as the primary indicator of this compound treatment efficacy. [, , , , , , , , , , , , , , ] The research does not delve into specific biomarkers to predict treatment response or identify potential adverse effects.
Q16: What analytical methods are used to quantify this compound?
A19: Several analytical techniques have been employed for this compound quantification, including high-performance liquid chromatography (HPLC) and UV spectrophotometry. [, , , , , ] Stability indicating HPTLC methods have also been developed to assess this compound stability. []
Q17: Are there specific methods for analyzing this compound in biological samples?
A17: The provided research does not delve into specific details regarding the analysis of this compound in biological samples.
Q18: What is the environmental impact of this compound?
A18: The provided research focuses on the clinical aspects of this compound, and information regarding its environmental impact and degradation is not discussed.
Q19: What quality control measures are in place for this compound manufacturing?
A19: Specific quality control and assurance measures implemented during this compound development, manufacturing, and distribution are not discussed in detail in the provided research.
Q20: Does this compound elicit any immune responses?
A20: The provided scientific literature primarily focuses on the antiviral efficacy and safety profile of this compound. Information concerning its potential immunogenicity and effects on immunological responses is not discussed.
Q21: Does this compound interact with drug transporters?
A26: Preliminary research suggests that genetic variants in drug transporter genes like ABCB1 might affect this compound's primary metabolite concentration. [] This highlights the need for further investigation into potential drug-transporter interactions.
Q22: Does this compound affect drug-metabolizing enzymes?
A22: The provided research focuses on this compound's interaction with HCV and its clinical efficacy. It doesn't detail its impact on drug-metabolizing enzyme activity.
Q23: What is known about the biocompatibility of this compound?
A23: The primary focus of the research is this compound's clinical effectiveness and safety in treating HCV. Specific details regarding its biocompatibility and biodegradability are not discussed.
Q24: Are there any alternative therapies to this compound-based regimens for HCV?
A29: The landscape of HCV treatment has evolved significantly with the advent of direct-acting antivirals (DAAs). [, , , , , , ] Prior to DAAs, the standard of care involved interferon-based therapies, which were associated with significant side effects and lower efficacy. [, , , , , ] this compound, as a highly effective DAA, has largely replaced interferon-based therapies as the preferred treatment option for most patients.
Q25: Are there strategies for recycling or managing this compound waste?
A25: The research provided does not offer information on this compound waste management or recycling strategies.
Q26: What research tools and resources have been crucial in this compound research?
A26: While not explicitly stated, the research highlights the use of several key tools and resources, including:
- HCV replicons: Essential for evaluating this compound's antiviral activity and resistance profiling. [, , ]
- Clinical trial networks: Collaborative efforts across multiple research centers were crucial for conducting large-scale clinical trials. [, , , , , , , , , , , , , , ]
- Analytical techniques: Methods like HPLC and UV spectrophotometry are vital for this compound quantification and stability studies. [, , , , , ]
Q27: What were the major milestones in the development of this compound?
A27: Key milestones in this compound's development include:
- Clinical trials: Successful completion of Phase 2 and 3 clinical trials demonstrating high SVR rates across HCV genotypes. [, , , , , , , , , , , , , , ]
- Regulatory approval: FDA approval of this compound in 2013, marking a significant advancement in HCV treatment. [, ]
- Real-world effectiveness: Continued evaluation of this compound's efficacy and safety in broader patient populations. [, ]
Q28: How has this compound research fostered cross-disciplinary collaborations?
A28: The development of this compound has spurred collaborations across various scientific disciplines, including:
- Virology and Molecular Biology: Understanding HCV replication mechanisms and resistance pathways. [, , ]
- Medicinal Chemistry and Pharmacology: Designing, synthesizing, and evaluating the drug's pharmacokinetic and pharmacodynamic properties. [, , , , ]
- Clinical Medicine and Hepatology: Conducting clinical trials, evaluating efficacy and safety, and managing HCV-infected patients. [, , , , , , , , , , , , , , ]
- Analytical Chemistry: Developing and validating methods for this compound quantification and quality control. [, , , , , ]
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。