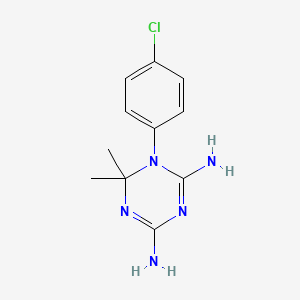
环胍乙啶
描述
环胍乙胺是一种二氢叶酸还原酶抑制剂,也是抗疟药伯氨喹的代谢产物。它主要负责伯氨喹的抗疟活性。
科学研究应用
环胍乙胺有几个科学研究应用,包括:
化学: 环胍乙胺被用作研究二氢叶酸还原酶抑制剂的模型化合物。
生物学: 环胍乙胺作为二氢叶酸还原酶抑制剂的作用使其在研究涉及叶酸代谢的细胞过程方面具有价值。
作用机制
环胍乙胺通过抑制二氢叶酸还原酶发挥作用,该酶对疟原虫中核酸的合成至关重要。 通过抑制这种酶,环胍乙胺破坏了寄生虫合成DNA和RNA的能力,最终导致其死亡 。 涉及的分子靶点和途径包括叶酸途径和核酸的合成 .
生化分析
Biochemical Properties
Cycloguanil plays a significant role in biochemical reactions, particularly in the inhibition of dihydrofolate reductase . This enzyme is crucial for the synthesis of nucleic acids, and its inhibition disrupts the reproduction of the malaria parasite . Cycloguanil interacts with this enzyme, leading to the disruption of deoxythymidylate synthesis .
Cellular Effects
Cycloguanil exerts its effects on various types of cells, particularly those infected by the malaria parasite. By inhibiting dihydrofolate reductase, it disrupts the parasite’s ability to reproduce within the host cell . This impacts cell function and can influence cell signaling pathways, gene expression, and cellular metabolism .
Molecular Mechanism
The molecular mechanism of Cycloguanil involves its binding to dihydrofolate reductase, inhibiting the enzyme and disrupting the synthesis of nucleic acids . This prevents the malaria parasite from reproducing within the host cell .
Metabolic Pathways
Cycloguanil is involved in the metabolic pathway related to the inhibition of dihydrofolate reductase . It interacts with this enzyme, disrupting the synthesis of nucleic acids and affecting metabolic flux and metabolite levels .
Transport and Distribution
Cycloguanil is transported and distributed within cells and tissues. It is known to be a substrate of organic cation transporters and multidrug and toxin extrusion proteins , which play a role in its distribution within cells.
准备方法
环胍乙胺可以通过多步合成过程合成然后将该中间体与丙酮缩合得到环胍乙胺 。工业生产方法可能包括优化反应条件以最大限度地提高产量和纯度。
化学反应分析
环胍乙胺会发生各种化学反应,包括:
氧化: 环胍乙胺在特定条件下可以被氧化,导致形成不同的氧化产物。
还原: 还原反应可以将环胍乙胺转化为其还原形式。
取代: 环胍乙胺可以发生取代反应,其中特定的官能团被其他官能团取代。
这些反应中常用的试剂和条件包括氧化剂、还原剂和催化剂。 这些反应形成的主要产物取决于所使用的特定试剂和条件 .
相似化合物的比较
环胍乙胺在结构上类似于其他二氢叶酸还原酶抑制剂,例如甲氧苄啶和甲氨蝶呤。环胍乙胺在针对疟原虫的特定活性方面是独一无二的。类似的化合物包括:
甲氧苄啶: 另一种用于抗疟治疗的二氢叶酸还原酶抑制剂。
甲氨蝶呤: 一种用于癌症治疗的二氢叶酸还原酶抑制剂.
属性
IUPAC Name |
1-(4-chlorophenyl)-6,6-dimethyl-1,3,5-triazine-2,4-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
QMNFFXRFOJIOKZ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1(N=C(N=C(N1C2=CC=C(C=C2)Cl)N)N)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C11H14ClN5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
152-53-4 (hydrochloride) | |
| Record name | Cycloguanil | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000516212 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID9022867 | |
| Record name | Cycloguanil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022867 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
251.71 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
516-21-2 | |
| Record name | Cycloguanil | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=516-21-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Cycloguanil | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000516212 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Cycloguanil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB14763 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cycloguanil | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022867 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | CYCLOGUANIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/26RM326WVN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: Cycloguanil inhibits the enzyme dihydrofolate reductase (DHFR) in the malaria parasite Plasmodium falciparum. [, , , ] This enzyme is crucial for the parasite's folate metabolism, which in turn is essential for DNA synthesis and cell division. [, , ]
A: By blocking DHFR, cycloguanil disrupts the synthesis of tetrahydrofolic acid, a coenzyme vital for the synthesis of purines and pyrimidines, the building blocks of DNA. [, ] This ultimately leads to the death of the parasite. [, ] Research has also shown that cycloguanil can impact downstream signaling pathways, such as STAT3 transcriptional activity, further contributing to its antimalarial effects. []
A: While primarily known for its activity against malaria parasites, cycloguanil also demonstrates inhibitory activity against Trypanosoma brucei PTR1 (TbPTR1), the enzyme pteridine reductase. [] This suggests potential as a dual PTR and DHFR inhibitor for treating human African trypanosomiasis. []
ANone: The provided research papers focus primarily on the biological activity and pharmacokinetic properties of cycloguanil. Information regarding its material compatibility and stability under various conditions is not extensively discussed.
ANone: Cycloguanil itself doesn't possess catalytic properties. It functions as an enzyme inhibitor rather than a catalyst. It is primarily used for its antimalarial properties due to its inhibitory effect on DHFR.
A: Yes, computational techniques like molecular docking and quantitative structure-activity relationship (QSAR) studies have been employed. [, ] These methods help predict the binding affinities of cycloguanil analogs with wild-type and mutant P. falciparum DHFR. [, ] Researchers have used programs like GOLD, FlexX, Glide, and Molegro to analyze and visualize interactions with the DHFR active site. []
A: Molecular docking studies suggest that P. falciparum DHFR exhibits stereoselectivity when binding to R and S enantiomers of cycloguanil analogs. [] Cycloguanil analogs with alkyl chain substituents favor binding to the R enantiomer as it minimizes steric hindrance with the Phe58 residue in the DHFR active site. [] Conversely, analogs with phenol chain substituents prefer the S enantiomer, avoiding steric clashes with Leu46 and Met55. []
ANone: The provided scientific research papers primarily focus on characterizing the mechanism of action, resistance patterns, and structure-activity relationships of cycloguanil and its analogs. They do not extensively cover aspects such as SHE regulations, detailed toxicology profiles, drug delivery strategies, environmental impact, or other points mentioned in questions 8-26.
A: Researchers frequently use in vitro drug susceptibility tests, often employing isotopic methods, to assess the efficacy of cycloguanil against Plasmodium falciparum. [, , , , ] These tests involve culturing the parasite in the presence of varying concentrations of cycloguanil and measuring its growth inhibition. [, , , , ] The concentration at which parasite growth is inhibited by 50% (IC50) is often used to compare the drug sensitivity of different parasite isolates. [, , , , ]
A: Yes, the composition of the culture medium can significantly influence the in vitro activity of cycloguanil. [] Studies have demonstrated that standard RPMI medium or RPMI with low concentrations of folate and para-aminobenzoic acid leads to higher IC50 values for cycloguanil compared to folate and para-aminobenzoic acid-free RPMI. [] These findings highlight the importance of carefully controlling for medium composition when performing in vitro drug susceptibility assays.
ANone: While the provided research focuses on specific aspects of cycloguanil's activity and resistance, detailed toxicological data and long-term safety profiles are not extensively discussed.
ANone: The provided research papers predominantly concentrate on the mechanisms of action, resistance patterns, and structure-activity relationships of cycloguanil and its analogs. Information regarding drug delivery, biomarkers, detailed analytical techniques, environmental impact, or other points mentioned in questions 13-26 is limited within these specific research papers.
A: Cycloguanil has a history dating back to the mid-20th century, emerging as a key player in antimalarial research. [] Initially, it was observed that the antimalarial activity of the prodrug chloroguanide hydrochloride was largely attributed to its dihydrotriazine metabolite, later identified as cycloguanil. [] This led to the development of cycloguanil pamoate, a poorly water-soluble salt form designed for intramuscular depot administration, to achieve prolonged antimalarial effects. []
A: Research on cycloguanil demonstrates the synergy between various scientific disciplines, including parasitology, pharmacology, medicinal chemistry, and computational chemistry. [, ] Understanding the mechanism of action, resistance mechanisms, and structure-activity relationships through both in vitro and in vivo studies has been crucial in guiding the development of more effective antimalarial therapies. [, ] The identification of cycloguanil as a potential inhibitor of Trypanosoma brucei PTR1 highlights the potential for cross-disciplinary applications in combating other parasitic diseases. []
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。