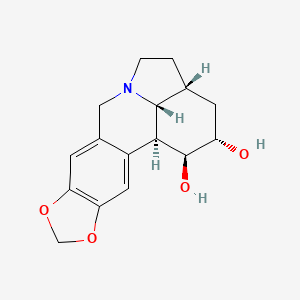
Dihydrolycorine
描述
二氢石蒜碱是一种从植物石蒜(Lycoris radiata)中提取的生物碱。它是石蒜碱的衍生物,石蒜碱以其各种药理作用而闻名。 二氢石蒜碱因其潜在的治疗应用而被研究,特别是在心血管保护、神经保护和抗肿瘤活性方面 .
作用机制
二氢石蒜碱通过各种分子靶点和途径发挥其作用:
心血管保护: 它抑制 Runx1 的表达,Runx1 是一个与心脏重塑不良相关的基因。
生化分析
Biochemical Properties
Dihydrolycorine plays a significant role in biochemical reactions by interacting with various enzymes, proteins, and other biomolecules. It has been shown to inhibit methoxamine-induced contraction of isolated rabbit aortic rings and rat anococcygeus muscle . Additionally, this compound interacts with fibrotic genes such as collagen I, TGF β, and p-smad3, as well as apoptosis-related genes like Bax and Bcl-2 . These interactions suggest that this compound can modulate cellular processes related to fibrosis and apoptosis.
Cellular Effects
This compound exerts notable effects on various cell types and cellular processes. In cardiomyocytes, it has been observed to reduce fibrosis and apoptosis, thereby improving cardiac function following myocardial infarction . This compound influences cell signaling pathways by downregulating Runx1, a gene associated with adverse cardiac remodeling . This regulation leads to improved cardiac contractile function and reduced cardiomyocyte hypertrophy.
Molecular Mechanism
At the molecular level, this compound exerts its effects through several mechanisms. It binds to Runx1, inhibiting its expression and activity . This inhibition results in the downregulation of fibrotic and apoptotic genes, thereby preventing adverse cardiac remodeling. Molecular docking and binding modeling studies have identified potential this compound-binding sites in Runx1, further elucidating its mechanism of action .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound have been studied over various time periods. It has been shown to maintain stability and efficacy in reducing cardiac fibrosis and dysfunction over extended periods . Long-term studies indicate that this compound can sustain its therapeutic effects, with minimal degradation observed in in vitro and in vivo models .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. At a dose of 80 mg/kg, this compound significantly reduces mean arterial pressure in normotensive rats . Higher doses have been associated with increased efficacy in reducing infarct size and cerebral edema in a rat model of focal cerebral ischemia-reperfusion injury . Toxic or adverse effects at high doses have not been extensively reported, suggesting a favorable safety profile.
Metabolic Pathways
This compound is involved in several metabolic pathways, interacting with enzymes and cofactors that modulate its activity. It is metabolized in the liver, where it undergoes biotransformation to produce active metabolites . These metabolites contribute to its overall pharmacological effects, influencing metabolic flux and metabolite levels in various tissues.
Transport and Distribution
Within cells and tissues, this compound is transported and distributed through specific transporters and binding proteins. It is known to accumulate in cardiac tissues, where it exerts its therapeutic effects . The distribution of this compound is influenced by factors such as blood flow, tissue binding, and membrane permeability .
Subcellular Localization
This compound localizes to specific subcellular compartments, where it interacts with target biomolecules. It has been observed to accumulate in the cytoplasm and nucleus of cardiomyocytes, where it modulates gene expression and cellular function . The subcellular localization of this compound is directed by targeting signals and post-translational modifications that ensure its proper distribution within the cell .
准备方法
二氢石蒜碱通过石蒜碱的氢化合成。该过程涉及在催化剂(通常为碳载钯(Pd/C))存在下,用氢气还原石蒜碱。 该反应在受控条件下进行,以确保选择性还原石蒜碱中的双键,从而形成二氢石蒜碱 .
化学反应分析
二氢石蒜碱会经历各种化学反应,包括:
氧化: 二氢石蒜碱可以被氧化形成相应的氧化物。常见的氧化剂包括高锰酸钾(KMnO₄)和过氧化氢(H₂O₂)。
还原: 如前所述,二氢石蒜碱是通过石蒜碱的还原形成的。进一步还原会导致形成更饱和的衍生物。
取代: 二氢石蒜碱可以发生取代反应,特别是在氮原子处。这些反应的常见试剂包括卤代烷烃和酰氯。
科学研究应用
心血管保护: 二氢石蒜碱已被证明通过在心肌梗死后下调 Runx1 来减轻心脏纤维化和功能障碍。
神经保护: 它通过减少局灶性脑缺血再灌注损伤模型中的梗塞面积、脑水肿和髓过氧化物酶活性来表现出神经保护活性.
相似化合物的比较
属性
IUPAC Name |
(1S,15R,17S,18S,19R)-5,7-dioxa-12-azapentacyclo[10.6.1.02,10.04,8.015,19]nonadeca-2,4(8),9-triene-17,18-diol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H19NO4/c18-11-3-8-1-2-17-6-9-4-12-13(21-7-20-12)5-10(9)14(15(8)17)16(11)19/h4-5,8,11,14-16,18-19H,1-3,6-7H2/t8-,11+,14+,15-,16-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VJILFEGOWCJNIK-MGRBZGILSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CN2CC3=CC4=C(C=C3C5C2C1CC(C5O)O)OCO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1CN2CC3=CC4=C(C=C3[C@H]5[C@H]2[C@H]1C[C@@H]([C@H]5O)O)OCO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H19NO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID701346488 | |
| Record name | Dihydrolycorine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID701346488 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
289.33 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
6271-21-2 | |
| Record name | Dihydrolycorine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0006271212 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dihydrolycorine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID701346488 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 6271-21-2 | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DIHYDROLYCORINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/Z7N4S72301 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。