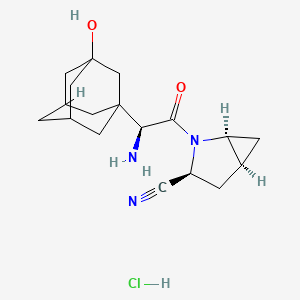
沙格列汀 hydrochloride
描述
Saxagliptin Hydrochloride is an orally active hypoglycemic drug used in the management of type 2 diabetes mellitus. It belongs to the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which work by increasing the levels of incretin hormones in the body. These hormones help regulate blood sugar levels by increasing insulin production and decreasing glucose production by the liver .
科学研究应用
沙格列汀盐酸盐具有广泛的科学研究应用:
化学: 它用于研究酶抑制剂及其对代谢途径的影响。
生物学: 它用于了解肠降血糖素激素在葡萄糖调节中的作用。
医学: 它主要用于治疗2型糖尿病,改善血糖控制。
工业: 它用于制药行业生产降糖药
生化分析
Biochemical Properties
Saxagliptin hydrochloride plays a crucial role in biochemical reactions by inhibiting the DPP-4 enzyme. This inhibition prevents the degradation of incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). By maintaining higher levels of these hormones, saxagliptin hydrochloride enhances insulin secretion and decreases glucagon release, leading to improved glycemic control . The compound interacts with the DPP-4 enzyme by forming a reversible, histidine-assisted covalent bond between its nitrile group and the serine residue in the active site of DPP-4 .
Cellular Effects
Saxagliptin hydrochloride influences various cellular processes, particularly in pancreatic beta cells. By inhibiting DPP-4, it increases the levels of GLP-1 and GIP, which in turn stimulate insulin secretion from beta cells. This leads to improved glucose uptake and utilization by cells, thereby lowering blood glucose levels . Additionally, saxagliptin hydrochloride has been shown to have minimal effects on cell signaling pathways and gene expression, making it a relatively safe option for long-term use .
Molecular Mechanism
The molecular mechanism of saxagliptin hydrochloride involves the inhibition of the DPP-4 enzyme. This enzyme is responsible for the degradation of incretin hormones. By inhibiting DPP-4, saxagliptin hydrochloride increases the half-life of GLP-1 and GIP, enhancing their biological effects . The compound forms a reversible covalent bond with the active site of DPP-4, which prevents the enzyme from degrading incretin hormones .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of saxagliptin hydrochloride have been observed over various time periods. The compound is stable and maintains its inhibitory effects on DPP-4 for up to 24 hours post-administration . Long-term studies have shown that saxagliptin hydrochloride remains effective in improving glycemic control without significant degradation or loss of potency .
Dosage Effects in Animal Models
In animal models, the effects of saxagliptin hydrochloride vary with different dosages. At lower doses, the compound effectively inhibits DPP-4 and improves glycemic control without adverse effects . At higher doses, saxagliptin hydrochloride may cause toxic effects, including gastrointestinal disturbances and potential hepatotoxicity . It is important to determine the optimal dosage to maximize therapeutic benefits while minimizing adverse effects.
Metabolic Pathways
Saxagliptin hydrochloride is primarily metabolized by the cytochrome P450 3A4/5 (CYP3A4/5) enzymes in the liver . The major metabolite, 5-hydroxy saxagliptin, retains some inhibitory activity against DPP-4 but is less potent than the parent compound . The metabolic pathways involve hydroxylation and subsequent conjugation reactions, leading to the formation of various metabolites that are excreted via urine and feces .
Transport and Distribution
Saxagliptin hydrochloride is well-absorbed and distributed throughout the body. It has a bioavailability of approximately 75% and reaches peak plasma concentrations within 2 hours of oral administration . The compound is primarily excreted through the urine, with a smaller portion eliminated via the bile . Saxagliptin hydrochloride does not significantly bind to plasma proteins, allowing for efficient distribution to target tissues .
Subcellular Localization
The subcellular localization of saxagliptin hydrochloride is primarily within the cytoplasm, where it interacts with the DPP-4 enzyme . The compound does not require specific targeting signals or post-translational modifications for its activity. Its inhibitory effects on DPP-4 are exerted directly within the cytoplasmic compartment, leading to increased levels of active incretin hormones .
准备方法
合成路线和反应条件: 沙格列汀盐酸盐的合成涉及几个关键步骤。一种常见的方法是在偶联试剂的存在下偶联两种氨基酸衍生物。 (S)-(+)-对甲苯磺酰胺、乙醇钛(IV)和金刚烷-1-甲醛在羟基苯并三唑和EDC (1-乙基-3-(3-二甲基氨基丙基)碳二亚胺)存在下的酰胺偶联是一个关键步骤 .
工业生产方法: 沙格列汀盐酸盐的工业生产通常采用高效液相色谱 (HPLC) 来定量和验证该化合物。 这种方法确保了最终产品的纯度和质量 .
化学反应分析
反应类型: 沙格列汀盐酸盐会发生各种化学反应,包括:
氧化: 此反应涉及添加氧或去除氢。
还原: 此反应涉及添加氢或去除氧。
取代: 此反应涉及用另一种原子或原子团取代一个原子或原子团。
常用试剂和条件: 这些反应中常用的试剂包括高锰酸钾等氧化剂、硼氢化钠等还原剂以及卤素等取代试剂。 这些反应的条件通常需要控制温度和pH值,以确保达到预期结果 .
主要产物: 这些反应产生的主要产物包括各种中间体,这些中间体对于沙格列汀盐酸盐的合成至关重要。 然后将这些中间体进一步加工以获得最终化合物 .
作用机制
沙格列汀盐酸盐通过抑制二肽基肽酶-4 (DPP-4) 酶发挥作用。这种抑制增加了肠降血糖素激素(如胰高血糖素样肽-1 (GLP-1) 和葡萄糖依赖性胰岛素促分泌多肽 (GIP))的水平。 这些激素通过增加胰岛素分泌和减少肝脏胰高血糖素分泌来帮助降低血糖水平 .
相似化合物:
二甲双胍: 另一种降糖药,可以提高胰岛素敏感性。
司美格鲁肽: 一种 GLP-1 受体激动剂,模拟肠降血糖素激素的作用。
比较:
沙格列汀盐酸盐与二甲双胍: 沙格列汀盐酸盐通过抑制 DPP-4 发挥作用,而二甲双胍则提高胰岛素敏感性。
沙格列汀盐酸盐与司美格鲁肽: 两种药物都提高肠降血糖素激素水平,但司美格鲁肽是 GLP-1 受体激动剂,而沙格列汀盐酸盐是 DPP-4 抑制剂。
沙格列汀盐酸盐因其独特的作用机制及其在治疗2型糖尿病的联合疗法中的有效性而脱颖而出。
相似化合物的比较
Metformin: Another antidiabetic drug that improves insulin sensitivity.
Semaglutide: A GLP-1 receptor agonist that mimics the effects of incretin hormones.
Comparison:
Saxagliptin Hydrochloride vs. Metformin: Saxagliptin Hydrochloride works by inhibiting DPP-4, while Metformin improves insulin sensitivity.
Saxagliptin Hydrochloride vs. Semaglutide: Both drugs increase the levels of incretin hormones, but Semaglutide is a GLP-1 receptor agonist, while Saxagliptin Hydrochloride is a DPP-4 inhibitor.
Saxagliptin Hydrochloride stands out due to its specific mechanism of action and its effectiveness in combination therapies for type 2 diabetes mellitus.
属性
IUPAC Name |
(1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxy-1-adamantyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile;hydrochloride | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H25N3O2.ClH/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17;/h10-15,23H,1-7,9,20H2;1H/t10?,11?,12-,13+,14+,15-,17?,18?;/m1./s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
TUAZNHHHYVBVBR-NHKADLRUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1C2CC2N(C1C#N)C(=O)C(C34CC5CC(C3)CC(C5)(C4)O)N.Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1[C@@H]2C[C@@H]2N([C@@H]1C#N)C(=O)[C@H](C34CC5CC(C3)CC(C5)(C4)O)N.Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H26ClN3O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID50991191 | |
| Record name | Saxagliptin hydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID50991191 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
351.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
709031-78-7 | |
| Record name | Saxagliptin hydrochloride | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0709031787 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Saxagliptin hydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID50991191 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile hydrochloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | SAXAGLIPTIN HYDROCHLORIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/Z8J84YIX6L | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
A: Saxagliptin Hydrochloride functions as a dipeptidyl peptidase-4 (DPP-4) inhibitor. [, , , ] This enzyme typically breaks down incretin hormones like glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). By inhibiting DPP-4, Saxagliptin Hydrochloride increases the levels of these incretin hormones. This, in turn, stimulates insulin secretion from pancreatic β-cells and suppresses glucagon release, ultimately leading to improved glycemic control. [, , ]
A: Saxagliptin Hydrochloride is represented by the molecular formula C18H25N3O2·HCl. Its molecular weight is 351.87 g/mol. [, ]
A: Several spectroscopic methods prove valuable in the analysis of Saxagliptin Hydrochloride. These include: * X-ray powder diffraction (XRPD): This technique is used to identify different crystalline forms of Saxagliptin Hydrochloride, such as the dihydrate form. [, ] * Fourier transform infrared spectroscopy (FTIR): FTIR aids in confirming the identity of Saxagliptin Hydrochloride and can also be used to study drug-excipient compatibility. [, , ] * UV-Vis Spectrophotometry: This technique is commonly employed for quantitative analysis of Saxagliptin Hydrochloride, often in conjunction with HPLC. [, , , , ]
A: Saxagliptin Hydrochloride exhibits instability due to a tendency for intra-molecular cyclization, forming a cyclicamidine. [] Careful selection of excipients during formulation is crucial to minimize this degradation pathway. [, ] Studies have shown compatibility with excipients like Methocel, Polyethylene Glycol (PEG), and various Opadry polymers. []
A: While the provided research does not offer a detailed SAR analysis, it is understood that even minor structural changes to Saxagliptin Hydrochloride could alter its binding affinity to DPP-4, thereby affecting its potency and selectivity. [, ] Further research in this area would be valuable.
A: The inherent instability of Saxagliptin Hydrochloride, particularly its susceptibility to cyclization, poses a significant formulation challenge. [, ] To enhance its stability and bioavailability, various strategies are employed, including the use of suitable excipients, controlled release formulations, and potentially, alternative routes of administration like buccal delivery. [, , , ]
A: Saxagliptin Hydrochloride undergoes significant hepatic metabolism. []
A: It possesses an elimination half-life of approximately 2.5 hours. [] This relatively short half-life necessitates regular dosing to maintain therapeutic levels.
A: Yes, research supports the efficacy of Saxagliptin Hydrochloride in managing Type 2 Diabetes. [, ] While specific details on preclinical models and clinical trials are not provided in the research excerpts, it is understood that the compound has been tested in both settings.
A: While the research excerpts do not specifically mention targeted delivery systems for Saxagliptin Hydrochloride, the development of buccal patches for this drug suggests exploration of alternative routes of administration. [] This could potentially enhance its bioavailability and efficacy. Further research on targeted delivery systems for this drug could be beneficial.
A: Several analytical techniques are employed for the quantification of Saxagliptin Hydrochloride.
* High-performance liquid chromatography (HPLC): HPLC, often coupled with UV detection, is a widely used method for the separation and quantification of Saxagliptin Hydrochloride, both alone and in combination with other drugs like Metformin Hydrochloride and Dapagliflozin. [, , , , , , ] * UV-Vis Spectrophotometry: This technique, frequently used in conjunction with HPLC, provides a simple and rapid method for quantitative analysis. [, , , ] * High-performance thin-layer chromatography (HPTLC): HPTLC offers a valuable alternative for analyzing Saxagliptin Hydrochloride in pharmaceutical preparations, especially in combination with other antidiabetic agents. []
A: The dissolution rate of Saxagliptin Hydrochloride is a critical factor influencing its bioavailability. [, ] Faster dissolution generally translates to better absorption and potentially higher efficacy. Formulation strategies often aim to enhance the dissolution rate of the drug. []
A: Analytical methods employed for Saxagliptin Hydrochloride analysis are rigorously validated according to ICH (International Conference on Harmonization) guidelines. [, , , ] Key validation parameters include: * Linearity: Assessing the method's ability to generate a linear response over a specific concentration range. * Accuracy: Determining how close the measured values are to the true value. * Precision: Evaluating the reproducibility of the method, often expressed as relative standard deviation. * Specificity: Confirming the method's ability to differentiate Saxagliptin Hydrochloride from other components in the sample. * Robustness: Examining the method's resilience to deliberate variations in analytical parameters.
A: The introduction of Saxagliptin Hydrochloride marked a significant advancement in Type 2 Diabetes treatment. [, ] As a highly selective DPP-4 inhibitor, it offered a novel mechanism for improving glycemic control with a generally favorable safety profile. Its development underscores the ongoing pursuit of more effective and well-tolerated therapies for this chronic condition.
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。



![N-(2,4-difluorophenyl)-2-[4-[[2-(4-ethylsulfonylphenyl)acetyl]amino]-2-fluorophenyl]-2-methylpropanamide](/img/structure/B610623.png)
![3-(1H-indol-2-yl)-2-[[5-[2-(4-methylphenyl)ethynyl]thiophen-2-yl]sulfonylamino]propanoic acid](/img/structure/B610628.png)