Glibenclamid
Übersicht
Beschreibung
Wissenschaftliche Forschungsanwendungen
Glibensamid hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung:
5. Wirkmechanismus
Glibensamid übt seine hypoglykämischen Wirkungen aus, indem es an die ATP-sensitiven Kaliumkanäle auf Pankreas-β-Zellen bindet und diese hemmt. Diese Hemmung führt zur Schließung dieser Kanäle, was zu einer Depolarisation der Zellmembran und der anschließenden Öffnung spannungsgesteuerter Calciumkanäle führt. Der Einstrom von Calciumionen löst die Freisetzung von Insulin aus den β-Zellen aus, wodurch der Blutzuckerspiegel gesenkt wird .
Ähnliche Verbindungen:
Einzigartigkeit von Glibensamid: Glibensamid ist einzigartig unter den Sulfonylharnstoffen aufgrund seines ausgeglichenen pharmakokinetischen Profils, das eine verlängerte Wirkungsdauer bietet und gleichzeitig ein relativ geringes Risiko für Hypoglykämie aufweist. Sein dualer Ausscheidungsweg (Urin und Fäkalien) unterscheidet es auch von anderen Sulfonylharnstoffen .
Wirkmechanismus
Target of Action
Glyburide, a second-generation sulfonylurea, primarily targets the ATP-sensitive potassium channels on pancreatic beta cells . These channels are known as sulfonylurea receptor 1 (SUR1) .
Mode of Action
Glyburide acts by closing the ATP-sensitive potassium channels on the pancreatic beta cells . This action leads to an increase in intracellular potassium and calcium ion concentrations . The rise in these ion concentrations stimulates the secretion of insulin, a hormone crucial for regulating blood sugar levels .
Biochemical Pathways
The primary biochemical pathway affected by Glyburide is the insulin signaling pathway. By stimulating the secretion of insulin, Glyburide enhances the body’s ability to regulate blood glucose levels . This results in a decrease in blood sugar levels, thereby helping to manage type 2 diabetes mellitus .
Pharmacokinetics
Glyburide is significantly absorbed within one hour of administration . It reaches peak drug levels at about four hours, and low but detectable levels can be found in the body up to twenty-four hours after administration . Glyburide is metabolized in the liver via the CYP450 system, specifically as a CYP2C9 substrate . The drug has a half-life of 10 hours and is excreted in the bile (50%) and urine (50%) as metabolites .
Result of Action
The primary result of Glyburide’s action is a reduction in blood sugar levels. By causing the pancreas to produce insulin and helping the body use insulin efficiently, Glyburide lowers blood sugar levels . This medication is particularly effective in individuals whose bodies naturally produce insulin .
Action Environment
The action, efficacy, and stability of Glyburide can be influenced by various environmental factors. For instance, the drug’s absorption and metabolism can be affected by the individual’s diet and lifestyle. Additionally, certain medical conditions, such as liver or kidney disease, can impact how the body processes Glyburide . Therefore, it’s important for individuals taking Glyburide to maintain a healthy lifestyle and manage any co-existing medical conditions under the guidance of a healthcare professional.
Biochemische Analyse
Biochemical Properties
By inhibiting these channels, Glyburide causes an increase in intracellular potassium and calcium ion concentrations . This biochemical interaction triggers the release of insulin, thereby playing a crucial role in the regulation of blood glucose levels .
Cellular Effects
Glyburide exerts significant effects on various types of cells, most notably the beta cells in the pancreas. It stimulates insulin secretion through the closure of ATP-sensitive potassium channels on these beta cells . This action raises intracellular potassium and calcium ion concentrations, which in turn triggers the release of insulin . The increased insulin helps to lower blood glucose levels, thereby managing the symptoms of type 2 diabetes .
Molecular Mechanism
The molecular mechanism of Glyburide involves its binding to and inhibition of the ATP-sensitive potassium channels (K ATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1) in pancreatic beta cells . This inhibition causes cell membrane depolarization, opening voltage-dependent calcium channels . The influx of calcium ions triggers the release of insulin, which then acts to lower blood glucose levels .
Temporal Effects in Laboratory Settings
In laboratory settings, Glyburide has been observed to have a long duration of action as it is given once daily . Its effects on cellular function, such as the stimulation of insulin secretion, are therefore sustained over a long period
Dosage Effects in Animal Models
While specific studies on the dosage effects of Glyburide in animal models are limited, it is known that the drug’s effects can vary with different dosages. For instance, in a study on the brain and whole-body distribution of Glyburide, it was found that the drug’s distribution, metabolism, and elimination are greatly dependent on organic anion transporting polypeptide (OATP) activity .
Metabolic Pathways
Glyburide is metabolized mainly by CYP3A4, followed by CYP2C9, CYP2C19, CYP3A7, and CYP3A5 . These enzymes metabolize Glyburide to various metabolites, including 4-trans-hydroxycyclohexyl glyburide (M1), 4-cis-hydroxycyclohexyl glyburide (M2a), 3-cis-hydroxycyclohexyl glyburide (M2b), 3-trans-hydroxycyclohexyl glyburide (M3), 2-trans-hydroxycyclohexyl glyburide (M4), and ethylhydroxycyclohexyl glyburide (M5) .
Transport and Distribution
Glyburide’s transport and distribution within cells and tissues are greatly influenced by OATP activity . This transporter plays a critical role in the hepatic uptake of Glyburide, which is the first step in its hepatic clearance . Moreover, ATP-binding cassette (ABC) transporters, including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and probably multidrug resistance protein 4, work in synergy to limit Glyburide’s brain uptake .
Subcellular Localization
The subcellular localization of Glyburide is primarily within the cytoplasm of cells . It is transported into the cell via OATP and is then distributed within the cell to exert its effects
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Glyburide is synthesized through a multi-step process involving the reaction of 5-chloro-2-methoxybenzoic acid with 2-aminoethylsulfonamide to form 5-chloro-2-methoxy-N-(2-(4-sulfamoylphenyl)ethyl)benzamide. This intermediate is then reacted with cyclohexyl isocyanate to yield glyburide .
Industrial Production Methods: Industrial production of glyburide involves optimizing the reaction conditions to ensure high yield and purity. The process typically includes steps such as crystallization, filtration, and drying to obtain the final product in its pure form .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Glibensamid unterliegt verschiedenen chemischen Reaktionen, darunter Oxidation, Reduktion und Substitution.
Häufige Reagenzien und Bedingungen:
Oxidation: Glibensamid kann unter sauren Bedingungen mit Oxidationsmitteln wie Wasserstoffperoxid oder Kaliumpermanganat oxidiert werden.
Reduktion: Die Reduktion von Glibensamid kann mit Reduktionsmitteln wie Natriumborhydrid oder Lithiumaluminiumhydrid erreicht werden.
Substitution: Substitutionsreaktionen mit Glibensamid verwenden häufig Reagenzien wie Halogene oder Alkylierungsmittel.
Hauptprodukte, die gebildet werden: Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, hängen von den verwendeten Reagenzien und Bedingungen ab. Zum Beispiel kann die Oxidation hydroxylierte Derivate ergeben, während die Reduktion dechlorierte oder demethylierte Produkte erzeugen kann .
Vergleich Mit ähnlichen Verbindungen
Uniqueness of Glyburide: Glyburide is unique among sulfonylureas due to its balanced pharmacokinetic profile, which provides a prolonged duration of action while maintaining a relatively low risk of hypoglycemia. Its dual excretion pathway (urine and feces) also distinguishes it from other sulfonylureas .
Eigenschaften
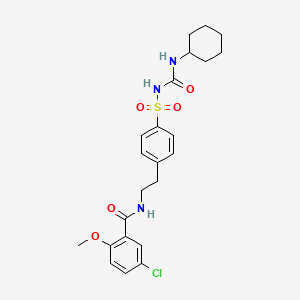
IUPAC Name |
5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-2-methoxybenzamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZNNLBTZKUZBEKO-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C(C=C(C=C1)Cl)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H28ClN3O5S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0037237 | |
| Record name | Glybenclamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0037237 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
494.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
2.06e-03 g/L | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Glyburide belongs to a class of drugs known as sulfonylureas. These drugs act by closing ATP-sensitive potassium channels on pancreatic beta cells. The ATP-sensitive potassium channels on beta cells are known as sulfonylurea receptor 1 (SUR1). Under low glucose concentrations, SUR1 remains open, allowing for potassium ion efflux to create a -70mV membrane potential. Normally SUR1 closes in response to high glucose concentrations, the membrane potential of the cells becomes less negative, the cell depolarizes, voltage gated calcium channels open, calcium ions enter the cell, and the increased intracellular calcium concentration stimulates the release of insulin containing granules. Glyburide bypasses this process by forcing SUR1 closed and stimulating increased insulin secretion. | |
| Record name | Glyburide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01016 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
10238-21-8 | |
| Record name | Glibenclamide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=10238-21-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Glyburide [USAN:USP] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0010238218 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Glyburide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01016 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | glyburide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759618 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Glybenclamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0037237 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Glibenclamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.030.505 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | GLYBURIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/SX6K58TVWC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
169 - 170 °C | |
| Record name | Glyburide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01016 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.