Glyburide
Overview
Description
Mechanism of Action
Target of Action
Glyburide, a second-generation sulfonylurea, primarily targets the ATP-sensitive potassium channels on pancreatic beta cells . These channels are known as sulfonylurea receptor 1 (SUR1) .
Mode of Action
This compound acts by closing the ATP-sensitive potassium channels on the pancreatic beta cells . This action leads to an increase in intracellular potassium and calcium ion concentrations . The rise in these ion concentrations stimulates the secretion of insulin, a hormone crucial for regulating blood sugar levels .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the insulin signaling pathway. By stimulating the secretion of insulin, this compound enhances the body’s ability to regulate blood glucose levels . This results in a decrease in blood sugar levels, thereby helping to manage type 2 diabetes mellitus .
Pharmacokinetics
This compound is significantly absorbed within one hour of administration . It reaches peak drug levels at about four hours, and low but detectable levels can be found in the body up to twenty-four hours after administration . This compound is metabolized in the liver via the CYP450 system, specifically as a CYP2C9 substrate . The drug has a half-life of 10 hours and is excreted in the bile (50%) and urine (50%) as metabolites .
Result of Action
The primary result of this compound’s action is a reduction in blood sugar levels. By causing the pancreas to produce insulin and helping the body use insulin efficiently, this compound lowers blood sugar levels . This medication is particularly effective in individuals whose bodies naturally produce insulin .
Action Environment
The action, efficacy, and stability of this compound can be influenced by various environmental factors. For instance, the drug’s absorption and metabolism can be affected by the individual’s diet and lifestyle. Additionally, certain medical conditions, such as liver or kidney disease, can impact how the body processes this compound . Therefore, it’s important for individuals taking this compound to maintain a healthy lifestyle and manage any co-existing medical conditions under the guidance of a healthcare professional.
Biochemical Analysis
Biochemical Properties
By inhibiting these channels, Glyburide causes an increase in intracellular potassium and calcium ion concentrations . This biochemical interaction triggers the release of insulin, thereby playing a crucial role in the regulation of blood glucose levels .
Cellular Effects
This compound exerts significant effects on various types of cells, most notably the beta cells in the pancreas. It stimulates insulin secretion through the closure of ATP-sensitive potassium channels on these beta cells . This action raises intracellular potassium and calcium ion concentrations, which in turn triggers the release of insulin . The increased insulin helps to lower blood glucose levels, thereby managing the symptoms of type 2 diabetes .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to and inhibition of the ATP-sensitive potassium channels (K ATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1) in pancreatic beta cells . This inhibition causes cell membrane depolarization, opening voltage-dependent calcium channels . The influx of calcium ions triggers the release of insulin, which then acts to lower blood glucose levels .
Temporal Effects in Laboratory Settings
In laboratory settings, this compound has been observed to have a long duration of action as it is given once daily . Its effects on cellular function, such as the stimulation of insulin secretion, are therefore sustained over a long period
Dosage Effects in Animal Models
While specific studies on the dosage effects of this compound in animal models are limited, it is known that the drug’s effects can vary with different dosages. For instance, in a study on the brain and whole-body distribution of this compound, it was found that the drug’s distribution, metabolism, and elimination are greatly dependent on organic anion transporting polypeptide (OATP) activity .
Metabolic Pathways
This compound is metabolized mainly by CYP3A4, followed by CYP2C9, CYP2C19, CYP3A7, and CYP3A5 . These enzymes metabolize this compound to various metabolites, including 4-trans-hydroxycyclohexyl this compound (M1), 4-cis-hydroxycyclohexyl this compound (M2a), 3-cis-hydroxycyclohexyl this compound (M2b), 3-trans-hydroxycyclohexyl this compound (M3), 2-trans-hydroxycyclohexyl this compound (M4), and ethylhydroxycyclohexyl this compound (M5) .
Transport and Distribution
This compound’s transport and distribution within cells and tissues are greatly influenced by OATP activity . This transporter plays a critical role in the hepatic uptake of this compound, which is the first step in its hepatic clearance . Moreover, ATP-binding cassette (ABC) transporters, including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and probably multidrug resistance protein 4, work in synergy to limit this compound’s brain uptake .
Subcellular Localization
The subcellular localization of this compound is primarily within the cytoplasm of cells . It is transported into the cell via OATP and is then distributed within the cell to exert its effects
Preparation Methods
Synthetic Routes and Reaction Conditions: Glyburide is synthesized through a multi-step process involving the reaction of 5-chloro-2-methoxybenzoic acid with 2-aminoethylsulfonamide to form 5-chloro-2-methoxy-N-(2-(4-sulfamoylphenyl)ethyl)benzamide. This intermediate is then reacted with cyclohexyl isocyanate to yield this compound .
Industrial Production Methods: Industrial production of this compound involves optimizing the reaction conditions to ensure high yield and purity. The process typically includes steps such as crystallization, filtration, and drying to obtain the final product in its pure form .
Chemical Reactions Analysis
Types of Reactions: Glyburide undergoes various chemical reactions, including oxidation, reduction, and substitution.
Common Reagents and Conditions:
Oxidation: this compound can be oxidized using reagents such as hydrogen peroxide or potassium permanganate under acidic conditions.
Reduction: Reduction of this compound can be achieved using reducing agents like sodium borohydride or lithium aluminum hydride.
Substitution: Substitution reactions involving this compound often use reagents like halogens or alkylating agents.
Major Products Formed: The major products formed from these reactions depend on the specific reagents and conditions used. For example, oxidation may yield hydroxylated derivatives, while reduction can produce dechlorinated or demethylated products .
Scientific Research Applications
Glyburide has a wide range of scientific research applications:
Comparison with Similar Compounds
Uniqueness of this compound: this compound is unique among sulfonylureas due to its balanced pharmacokinetic profile, which provides a prolonged duration of action while maintaining a relatively low risk of hypoglycemia. Its dual excretion pathway (urine and feces) also distinguishes it from other sulfonylureas .
Properties
IUPAC Name |
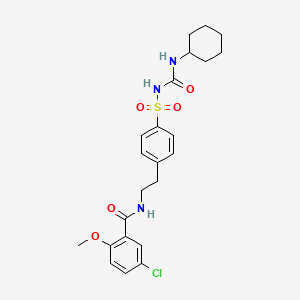
5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-2-methoxybenzamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZNNLBTZKUZBEKO-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C(C=C(C=C1)Cl)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H28ClN3O5S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0037237 | |
| Record name | Glybenclamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0037237 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
494.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
2.06e-03 g/L | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Glyburide belongs to a class of drugs known as sulfonylureas. These drugs act by closing ATP-sensitive potassium channels on pancreatic beta cells. The ATP-sensitive potassium channels on beta cells are known as sulfonylurea receptor 1 (SUR1). Under low glucose concentrations, SUR1 remains open, allowing for potassium ion efflux to create a -70mV membrane potential. Normally SUR1 closes in response to high glucose concentrations, the membrane potential of the cells becomes less negative, the cell depolarizes, voltage gated calcium channels open, calcium ions enter the cell, and the increased intracellular calcium concentration stimulates the release of insulin containing granules. Glyburide bypasses this process by forcing SUR1 closed and stimulating increased insulin secretion. | |
| Record name | Glyburide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01016 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
10238-21-8 | |
| Record name | Glibenclamide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=10238-21-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Glyburide [USAN:USP] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0010238218 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Glyburide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01016 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | glyburide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759618 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Glybenclamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0037237 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Glibenclamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.030.505 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | GLYBURIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/SX6K58TVWC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
169 - 170 °C | |
| Record name | Glyburide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01016 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.