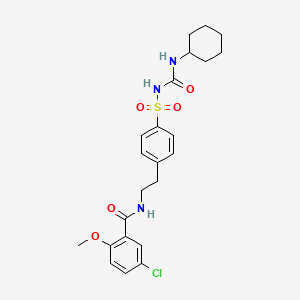
Gliburida
Descripción general
Descripción
Es un agente hipoglucémico oral que ayuda a controlar los niveles de azúcar en la sangre al estimular la liberación de insulina del páncreas . La glibúrida se introdujo por primera vez en la práctica clínica en 1969 y desde entonces se ha convertido en un medicamento ampliamente utilizado para el manejo de la diabetes .
Aplicaciones Científicas De Investigación
La glibúrida tiene una amplia gama de aplicaciones de investigación científica:
Mecanismo De Acción
La glibúrida ejerce sus efectos hipoglucémicos al unirse e inhibir los canales de potasio sensibles al ATP en las células beta pancreáticas. Esta inhibición conduce al cierre de estos canales, lo que resulta en la despolarización de la membrana celular y la posterior apertura de los canales de calcio dependientes de voltaje. La entrada de iones de calcio desencadena la liberación de insulina de las células beta, lo que reduce los niveles de glucosa en sangre .
Compuestos Similares:
Singularidad de la Glibúrida: La glibúrida es única entre las sulfonilureas debido a su perfil farmacocinético equilibrado, que proporciona una duración de acción prolongada al tiempo que mantiene un riesgo relativamente bajo de hipoglucemia. Su vía de excreción dual (orina y heces) también la distingue de otras sulfonilureas .
Análisis Bioquímico
Biochemical Properties
By inhibiting these channels, Glyburide causes an increase in intracellular potassium and calcium ion concentrations . This biochemical interaction triggers the release of insulin, thereby playing a crucial role in the regulation of blood glucose levels .
Cellular Effects
Glyburide exerts significant effects on various types of cells, most notably the beta cells in the pancreas. It stimulates insulin secretion through the closure of ATP-sensitive potassium channels on these beta cells . This action raises intracellular potassium and calcium ion concentrations, which in turn triggers the release of insulin . The increased insulin helps to lower blood glucose levels, thereby managing the symptoms of type 2 diabetes .
Molecular Mechanism
The molecular mechanism of Glyburide involves its binding to and inhibition of the ATP-sensitive potassium channels (K ATP) inhibitory regulatory subunit sulfonylurea receptor 1 (SUR1) in pancreatic beta cells . This inhibition causes cell membrane depolarization, opening voltage-dependent calcium channels . The influx of calcium ions triggers the release of insulin, which then acts to lower blood glucose levels .
Temporal Effects in Laboratory Settings
In laboratory settings, Glyburide has been observed to have a long duration of action as it is given once daily . Its effects on cellular function, such as the stimulation of insulin secretion, are therefore sustained over a long period
Dosage Effects in Animal Models
While specific studies on the dosage effects of Glyburide in animal models are limited, it is known that the drug’s effects can vary with different dosages. For instance, in a study on the brain and whole-body distribution of Glyburide, it was found that the drug’s distribution, metabolism, and elimination are greatly dependent on organic anion transporting polypeptide (OATP) activity .
Metabolic Pathways
Glyburide is metabolized mainly by CYP3A4, followed by CYP2C9, CYP2C19, CYP3A7, and CYP3A5 . These enzymes metabolize Glyburide to various metabolites, including 4-trans-hydroxycyclohexyl glyburide (M1), 4-cis-hydroxycyclohexyl glyburide (M2a), 3-cis-hydroxycyclohexyl glyburide (M2b), 3-trans-hydroxycyclohexyl glyburide (M3), 2-trans-hydroxycyclohexyl glyburide (M4), and ethylhydroxycyclohexyl glyburide (M5) .
Transport and Distribution
Glyburide’s transport and distribution within cells and tissues are greatly influenced by OATP activity . This transporter plays a critical role in the hepatic uptake of Glyburide, which is the first step in its hepatic clearance . Moreover, ATP-binding cassette (ABC) transporters, including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and probably multidrug resistance protein 4, work in synergy to limit Glyburide’s brain uptake .
Subcellular Localization
The subcellular localization of Glyburide is primarily within the cytoplasm of cells . It is transported into the cell via OATP and is then distributed within the cell to exert its effects
Métodos De Preparación
Rutas Sintéticas y Condiciones de Reacción: La glibúrida se sintetiza mediante un proceso de varios pasos que involucra la reacción del ácido 5-cloro-2-metoxibenzoico con 2-aminoetilsulfonamida para formar 5-cloro-2-metoxi-N-(2-(4-sulfamoylfenil)etil)benzamida. Este intermedio luego se hace reaccionar con isocianato de ciclohexilo para producir glibúrida .
Métodos de Producción Industrial: La producción industrial de glibúrida implica la optimización de las condiciones de reacción para garantizar un alto rendimiento y pureza. El proceso generalmente incluye pasos como cristalización, filtración y secado para obtener el producto final en su forma pura .
Análisis De Reacciones Químicas
Tipos de Reacciones: La glibúrida experimenta varias reacciones químicas, incluida la oxidación, reducción y sustitución.
Reactivos y Condiciones Comunes:
Oxidación: La glibúrida se puede oxidar utilizando reactivos como peróxido de hidrógeno o permanganato de potasio en condiciones ácidas.
Reducción: La reducción de la glibúrida se puede lograr utilizando agentes reductores como borohidruro de sodio o hidruro de litio y aluminio.
Sustitución: Las reacciones de sustitución que involucran glibúrida a menudo utilizan reactivos como halógenos o agentes alquilantes.
Principales Productos Formados: Los principales productos formados a partir de estas reacciones dependen de los reactivos y condiciones específicos utilizados. Por ejemplo, la oxidación puede producir derivados hidroxilados, mientras que la reducción puede producir productos desclorados o desmetilados .
Comparación Con Compuestos Similares
Uniqueness of Glyburide: Glyburide is unique among sulfonylureas due to its balanced pharmacokinetic profile, which provides a prolonged duration of action while maintaining a relatively low risk of hypoglycemia. Its dual excretion pathway (urine and feces) also distinguishes it from other sulfonylureas .
Propiedades
IUPAC Name |
5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-2-methoxybenzamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZNNLBTZKUZBEKO-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C(C=C(C=C1)Cl)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H28ClN3O5S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0037237 | |
| Record name | Glybenclamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0037237 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
494.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
2.06e-03 g/L | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Glyburide belongs to a class of drugs known as sulfonylureas. These drugs act by closing ATP-sensitive potassium channels on pancreatic beta cells. The ATP-sensitive potassium channels on beta cells are known as sulfonylurea receptor 1 (SUR1). Under low glucose concentrations, SUR1 remains open, allowing for potassium ion efflux to create a -70mV membrane potential. Normally SUR1 closes in response to high glucose concentrations, the membrane potential of the cells becomes less negative, the cell depolarizes, voltage gated calcium channels open, calcium ions enter the cell, and the increased intracellular calcium concentration stimulates the release of insulin containing granules. Glyburide bypasses this process by forcing SUR1 closed and stimulating increased insulin secretion. | |
| Record name | Glyburide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01016 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
10238-21-8 | |
| Record name | Glibenclamide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=10238-21-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Glyburide [USAN:USP] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0010238218 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Glyburide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01016 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | glyburide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759618 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Glybenclamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0037237 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Glibenclamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.030.505 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | GLYBURIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/SX6K58TVWC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
169 - 170 °C | |
| Record name | Glyburide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01016 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Glyburide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015151 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.