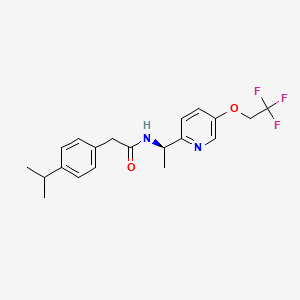
MK-8998
Übersicht
Beschreibung
Suvecaltamide is a highly selective modulator of T-type calcium channels, specifically targeting the Ca V 3.1, Ca V 3.2, and Ca V 3.3 subtypes . It is currently under investigation for its potential therapeutic effects in treating essential tremor, a progressive neurological disorder characterized by involuntary and rhythmic shaking .
Wissenschaftliche Forschungsanwendungen
Suvecaltamide hat verschiedene Anwendungen in der wissenschaftlichen Forschung:
5. Wirkmechanismus
Suvecaltamide übt seine Wirkung aus, indem es T-Typ-Kalziumkanäle selektiv moduliert. Diese Kanäle spielen eine entscheidende Rolle bei der Regulierung der Erregbarkeit und oszillatorischen Aktivität von Neuronen . Indem es an eine bestimmte Konformation des Kanals bindet und diese stabilisiert, reduziert Suvecaltamide die Aktivität dieser Kanäle, wodurch die Schwere des Tremors verringert und die Funktion bei Personen mit essentiellem Tremor verbessert wird .
Wirkmechanismus
Target of Action
The primary target of MK-8998 is the T-type calcium channel . These channels are low-voltage activated channels that contribute to the pacemaker activity in the heart and neurons. They play a crucial role in neuronal signaling and membrane transportation.
Mode of Action
This compound acts as a potent and selective inhibitor of the T-type calcium channel . By inhibiting these channels, this compound can modulate the flow of calcium ions, which are essential for the function of nerve cells and muscle cells.
Pharmacokinetics
It is known that the compound is soluble in dmso , which suggests that it may have good bioavailability
Result of Action
The inhibition of T-type calcium channels by this compound can have various molecular and cellular effects. For example, it can modulate neuronal signaling, which could potentially have therapeutic effects in conditions such as epilepsy and pain .
Vorbereitungsmethoden
The synthesis of Suvecaltamide involves several key steps:
Starting Materials: The synthesis begins with the preparation of 4-(1-methylethyl)-N-((1R)-1-(5-(2,2,2-trifluoroethoxy)-2-pyridinyl)ethyl)benzeneacetamide.
Reaction Conditions: The reaction conditions typically involve the use of organic solvents and catalysts to facilitate the formation of the desired compound.
Industrial Production: Industrial production methods for Suvecaltamide are designed to ensure high yield and purity.
Analyse Chemischer Reaktionen
Suvecaltamide durchläuft verschiedene chemische Reaktionen, darunter:
Oxidation: Suvecaltamide kann unter bestimmten Bedingungen oxidiert werden, um oxidierte Derivate zu bilden.
Häufige Reagenzien und Bedingungen, die bei diesen Reaktionen verwendet werden, umfassen Oxidationsmittel, Reduktionsmittel und Katalysatoren. Die Hauptprodukte, die aus diesen Reaktionen gebildet werden, hängen von den spezifischen Reaktionsbedingungen und den verwendeten Reagenzien ab .
Vergleich Mit ähnlichen Verbindungen
Suvecaltamide ist einzigartig in seiner hohen Selektivität und zustandsabhängigen Modulation von T-Typ-Kalziumkanälen . Ähnliche Verbindungen umfassen:
CX-8998: Ein weiterer T-Typ-Kalziumkanalmodulator mit ähnlichen Eigenschaften.
JZP-385: Eine Verbindung mit ähnlicher pharmakologischer Aktivität.
MK-8998: Eine weitere Verbindung, die auf T-Typ-Kalziumkanäle abzielt.
Diese Verbindungen teilen ähnliche Wirkmechanismen, können sich jedoch in ihrer Selektivität, Potenz und pharmakokinetischen Eigenschaften unterscheiden .
Eigenschaften
IUPAC Name |
2-(4-propan-2-ylphenyl)-N-[(1R)-1-[5-(2,2,2-trifluoroethoxy)pyridin-2-yl]ethyl]acetamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H23F3N2O2/c1-13(2)16-6-4-15(5-7-16)10-19(26)25-14(3)18-9-8-17(11-24-18)27-12-20(21,22)23/h4-9,11,13-14H,10,12H2,1-3H3,(H,25,26)/t14-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
IQIKXZMPPBEWAD-CQSZACIVSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C1=CC=C(C=C1)CC(=O)NC(C)C2=NC=C(C=C2)OCC(F)(F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H](C1=NC=C(C=C1)OCC(F)(F)F)NC(=O)CC2=CC=C(C=C2)C(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H23F3N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
380.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
953778-58-0 | |
| Record name | Suvecaltamide [USAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0953778580 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | SUVECALTAMIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/MIA4WMP8QN | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![7H-Pyrido[1,2,3-de]-1,4-benzothiazine-6-carboxylic acid, 9-fluoro-3-(fluoromethyl)-2,3-dihydro-10-(4-methyl-1-piperazinyl)-7-oxo-](/img/structure/B1676557.png)
![(1R,2R,3R,5R,6R)-2-amino-3-[(3,4-dichlorophenyl)methoxy]-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid](/img/structure/B1676573.png)
