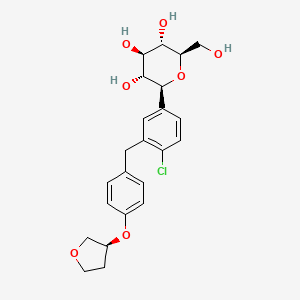
Empagliflozin
Übersicht
Beschreibung
Empagliflozin is an oral medication used primarily to manage type 2 diabetes mellitus. It belongs to the class of drugs known as sodium-glucose co-transporter-2 (SGLT2) inhibitors. By inhibiting SGLT2, this compound reduces glucose reabsorption in the kidneys, leading to increased glucose excretion through urine. This helps lower blood sugar levels in patients with type 2 diabetes .
Wissenschaftliche Forschungsanwendungen
Empagliflozin hat eine breite Palette von Anwendungen in der wissenschaftlichen Forschung, darunter:
Diabetesmanagement: This compound wird hauptsächlich zur Behandlung von Typ-2-Diabetes eingesetzt, indem es den Blutzuckerspiegel senkt
Herz-Kreislauf-Vorteile: Die Forschung hat gezeigt, dass this compound das Risiko von Herz-Kreislauf-Ereignissen wie Herzinfarkt und Schlaganfall bei Patienten mit Typ-2-Diabetes reduzieren kann
Herzinsuffizienz: Die Verbindung wird auch zur Behandlung von Herzinsuffizienz eingesetzt und bietet Vorteile, die über die Blutzuckerkontrolle hinausgehen.
5. Wirkmechanismus
This compound übt seine Wirkung aus, indem es den Natrium-Glukose-Cotransporter-2 (SGLT2) in den Nieren hemmt. Diese Hemmung reduziert die Rückresorption von Glukose aus dem Glomerulumfiltrat, was zu einer erhöhten Glukose-Ausscheidung im Urin führt. Die Senkung des Blutzuckerspiegels trägt zur Behandlung von Typ-2-Diabetes bei. Zusätzlich wurde gezeigt, dass this compound kardioprotektive und nephroprotektive Wirkungen hat, von denen angenommen wird, dass sie durch Mechanismen wie die Reduzierung von oxidativem Stress und die Verbesserung der Endothelfunktion vermittelt werden .
Wirkmechanismus
Target of Action
Empagliflozin primarily targets the sodium-glucose co-transporter-2 (SGLT2) . SGLT2 is the predominant transporter responsible for the reabsorption of glucose from the glomerular filtrate back into the circulation . It is primarily located in the kidney and plays a crucial role in maintaining glucose homeostasis .
Mode of Action
This compound acts as an inhibitor of SGLT2 . By inhibiting SGLT2, this compound prevents glucose reabsorption in the kidneys, thereby increasing the amount of glucose excreted in the urine . This leads to a reduction in blood glucose levels, which is beneficial for managing type 2 diabetes mellitus .
Biochemical Pathways
This compound’s action on SGLT2 affects several biochemical pathways. One of the key pathways involves the NLRP3 inflammasome and MyD88-related pathways . Inhibition of these pathways by this compound has been associated with anti-apoptotic and anti-fibrotic effects . Additionally, this compound has been shown to impact pathways related to cardiomyocyte contraction/relaxation, iron handling, and other metabolic and renal targets .
Result of Action
This compound has several molecular and cellular effects. It has been shown to ameliorate myocardial extracellular matrix remodeling, cardiomyocyte stiffness, and concentric hypertrophy . Additionally, this compound has been found to inhibit microglia activation and neuroinflammation resulting from injury . It also improves pancreatic beta-cell function by reducing pancreatic beta-cell burden and glucotoxicity .
Action Environment
The action of this compound can be influenced by various environmental factors. For instance, renal function can impact the efficacy of this compound, as its mechanism of action is contingent on the renal excretion of glucose . Therefore, this compound may be held in cases of acute kidney injury . Furthermore, the drug’s blood pressure-lowering effects have been associated with its action in non-diabetic hypertensive rats .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: Empagliflozin is synthesized through a multi-step process involving several key intermediates. One common synthetic route involves the following steps:
Formation of Intermediate I: The initial step involves the reaction of 4-chloro-3-(4-(tetrahydrofuran-3-yloxy)benzyl)phenyl with a suitable glucosyl donor to form Intermediate I.
Formation of Intermediate II: Intermediate I undergoes further reactions, including protection and deprotection steps, to yield Intermediate II.
Final Coupling: Intermediate II is then coupled with a suitable glucosyl donor under basic conditions to form this compound
Industrial Production Methods: Industrial production of this compound typically involves large-scale synthesis using optimized reaction conditions to ensure high yield and purity. The process includes:
Refluxing: The reaction mixture is refluxed to facilitate the formation of the desired product.
Purification: The crude product is purified using techniques such as recrystallization and chromatography to obtain this compound with high purity
Analyse Chemischer Reaktionen
Arten von Reaktionen: Empagliflozin unterliegt verschiedenen chemischen Reaktionen, darunter:
Hydrolyse: Die Verbindung ist besonders unter sauren und basischen Bedingungen anfällig für Hydrolyse.
Häufige Reagenzien und Bedingungen:
Oxidative Degradation: Reagenzien wie Wasserstoffperoxid oder andere Oxidationsmittel können eine oxidative Degradation induzieren.
Hauptprodukte, die gebildet werden:
Oxidative Abbauprodukte: Es können verschiedene Oxidationsprodukte gebildet werden, abhängig von den spezifischen Bedingungen und Reagenzien, die verwendet werden.
Hydrolyseprodukte: Hydrolyse kann zur Bildung kleinerer Fragmente und Abbauprodukte führen.
Vergleich Mit ähnlichen Verbindungen
Empagliflozin gehört zur Klasse der SGLT2-Inhibitor-Medikamente. Andere ähnliche Verbindungen in dieser Klasse sind:
Canagliflozin: Ein weiterer SGLT2-Inhibitor, der zur Behandlung von Typ-2-Diabetes eingesetzt wird.
Dapagliflozin: Diese Verbindung wird ebenfalls zur Behandlung von Typ-2-Diabetes eingesetzt und wurde für die Anwendung bei Herzinsuffizienz zugelassen.
Ertugliflozin: Ein weiterer SGLT2-Inhibitor mit ähnlichen Wirkmechanismen, aber unterschiedlichen pharmakokinetischen Eigenschaften.
Einzigartigkeit von this compound: this compound ist einzigartig in seiner hohen Selektivität für SGLT2 gegenüber SGLT1, was zu seinem Wirksamkeits- und Sicherheitsprofil beiträgt. Es wurde auch gezeigt, dass es signifikante kardiovaskuläre und renale Vorteile bietet, was es zu einer wertvollen Option für Patienten mit Typ-2-Diabetes und komorbiden Erkrankungen macht .
Eigenschaften
IUPAC Name |
(2S,3R,4R,5S,6R)-2-[4-chloro-3-[[4-[(3S)-oxolan-3-yl]oxyphenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H27ClO7/c24-18-6-3-14(23-22(28)21(27)20(26)19(11-25)31-23)10-15(18)9-13-1-4-16(5-2-13)30-17-7-8-29-12-17/h1-6,10,17,19-23,25-28H,7-9,11-12H2/t17-,19+,20+,21-,22+,23-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OBWASQILIWPZMG-QZMOQZSNSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1COCC1OC2=CC=C(C=C2)CC3=C(C=CC(=C3)C4C(C(C(C(O4)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1COC[C@H]1OC2=CC=C(C=C2)CC3=C(C=CC(=C3)[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H27ClO7 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID601026093 | |
| Record name | Empagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601026093 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
450.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
The vast majority of glucose filtered through the glomerulus is reabsorbed within the proximal tubule, primarily via SGLT2 (sodium-glucose linked co-transporter-2) which is responsible for ~90% of the total glucose reabsorption within the kidneys. Na+/K+-ATPase on the basolateral membrane of proximal tubular cells utilize ATP to actively pump Na+ ions into the interstitium surrounding the tubule, establishing a Na+ gradient within the tubular cell. SGLT2 on the apical membrane of these cells then utilize this gradient to facilitate secondary active co-transport of both Na+ and glucose out of the filtrate, thereby reabsorbing glucose back into the blood – inhibiting this co-transport, then, allows for a marked increase in glucosuria and decrease in blood glucose levels. Empagliflozin is a potent inhibitor of renal SGLT2 transporters located in the proximal tubules of the kidneys and works to lower blood glucose levels via an increase in glucosuria. Empagliflozin also appears to exert cardiovascular benefits - specifically in the prevention of heart failure - independent of its blood glucose-lowering effects, though the exact mechanism of this benefit is not precisely understood. Several theories have been posited, including the potential inhibition of Na+/H+ exchanger (NHE) 1 in the myocardium and NHE3 in the proximal tubule, reduction of pre-load via diuretic/natriuretic effects and reduction of blood pressure, prevention of cardiac fibrosis via suppression of pro-fibrotic markers, and reduction of pro-inflammatory adipokines. | |
| Record name | Empagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09038 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
864070-44-0 | |
| Record name | Empagliflozin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=864070-44-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Empagliflozin [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0864070440 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Empagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09038 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Empagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601026093 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-((S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | EMPAGLIFLOZIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/HDC1R2M35U | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![1-Ethyl-4-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-(1-ethylpyridin-1-ium-4-yl)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]pyridin-1-ium;dibromide](/img/structure/B1684252.png)
![2-[3-Cyano-5-(3,4-dichlorophenyl)-4,5-dimethylfuran-2-ylidene]propanedinitrile](/img/structure/B1684258.png)
