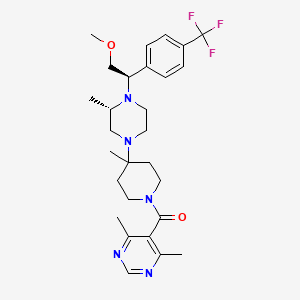
Vicriviroc
Übersicht
Beschreibung
Vicriviroc ist eine Pyrimidin-basierte Verbindung, die als CCR5-Eintrittsinhibitor von HIV-1 wirkt. Sie wurde von dem Pharmaunternehmen Schering-Plough entwickelt und hinsichtlich ihres Potenzials zur Behandlung von HIV-1-Infektionen untersucht . This compound hemmt die Wechselwirkung von HIV-1 mit dem CCR5-Rezeptor und verhindert so den Eintritt des Virus in Wirtszellen .
Wissenschaftliche Forschungsanwendungen
Wirkmechanismus
This compound wirkt als nicht-kompetitiver allosterischer Antagonist des CCR5-Rezeptors. Es bindet an eine hydrophobe Tasche zwischen den Transmembranhelices in der Nähe der extrazellulären Oberfläche des CCR5-Rezeptors. Diese Bindung bewirkt eine Konformationsänderung des Rezeptors, die die Bindung des HIV-1-gp120-Proteins an CCR5 verhindert. Dadurch wird der Eintritt von HIV-1 in Wirtszellen gehemmt und die frühen Stadien des viralen Lebenszyklus blockiert .
Wirkmechanismus
Target of Action
Vicriviroc primarily targets the C-C chemokine receptor type 5 (CCR5) . CCR5 is a protein on the surface of white blood cells that is involved in the immune system as it acts as a receptor for chemokines. This receptor is also utilized by HIV to enter and infect host cells .
Mode of Action
This compound is a noncompetitive allosteric antagonist of CCR5 . It binds to a hydrophobic pocket between transmembrane helices by the extracellular side of CCR5 . This binding induces a conformational change in the protein, which prevents the binding of gp120, a glycoprotein on the HIV envelope, to CCR5 . This action effectively blocks the entry of HIV into the cell .
Biochemical Pathways
The primary biochemical pathway affected by this compound is the HIV life cycle . By preventing the interaction of HIV-1 with CCR5, this compound inhibits the initial stages of the virus life cycle, specifically the entry of the virus into the host cell . This disruption in the life cycle of the virus prevents further infection and replication of HIV within the host.
Pharmacokinetics
It is known that this compound is metabolized primarily by theCYP3A4 system . The absorption, volume of distribution, protein binding, route of elimination, half-life, and clearance of this compound are yet to be determined .
Result of Action
The molecular effect of this compound’s action is the prevention of HIV entry into the host cell . On a cellular level, this results in a decrease in the viral load and an increase in CD4 cell counts, which are crucial for a healthy immune response .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen
Die Synthese von Vicriviroc erfolgt in mehreren Schritten, beginnend mit der Herstellung wichtiger Zwischenprodukte. Spezifische Reaktionsbedingungen, wie Temperatur, Lösungsmittel und Katalysatoren, werden optimiert, um eine hohe Ausbeute und Reinheit zu gewährleisten .
Industrielle Produktionsmethoden
Die industrielle Produktion von this compound folgt ähnlichen Synthesewegen, wird aber zur Deckung des kommerziellen Bedarfs hochskaliert. Der Prozess umfasst strenge Qualitätskontrollmaßnahmen, um Konsistenz und Einhaltung der behördlichen Standards sicherzustellen. Moderne Techniken wie kontinuierliche Durchflusschemie und automatisierte Synthese können eingesetzt werden, um die Effizienz zu steigern und die Produktionskosten zu senken .
Analyse Chemischer Reaktionen
Arten von Reaktionen
Vicriviroc unterliegt verschiedenen chemischen Reaktionen, darunter:
Oxidation: This compound kann oxidiert werden, um this compound-N-oxid zu bilden, einen wichtigen Metaboliten.
Reduktion: Reduktionsreaktionen können verwendet werden, um bestimmte funktionelle Gruppen im Molekül zu modifizieren.
Substitution: Substitutionsreaktionen können verwendet werden, um Substituenten am Pyrimidin-Kern einzuführen oder zu ersetzen.
Häufige Reagenzien und Bedingungen
Häufig verwendete Reagenzien in diesen Reaktionen umfassen Oxidationsmittel wie Cytochrom-P450-Enzyme für die Oxidation, Reduktionsmittel für die Reduktion und Nukleophile für Substitutionsreaktionen. Reaktionsbedingungen wie Temperatur, pH-Wert und Lösungsmittelwahl werden sorgfältig kontrolliert, um die gewünschten Ergebnisse zu erzielen .
Wichtigste gebildete Produkte
Zu den wichtigsten Produkten, die aus diesen Reaktionen entstehen, gehören this compound-N-oxid und andere Metaboliten, die aus der Modifikation der Stammverbindung resultieren .
Vergleich Mit ähnlichen Verbindungen
Vicriviroc gehört zu einer Klasse von Verbindungen, die als CCR5-Inhibitoren bekannt sind. Zu ähnlichen Verbindungen gehören:
Aplaviroc: Ein CCR5-Inhibitor, der aufgrund von Hepatotoxizitätsbedenken eingestellt wurde.
INCB009471: Ein CCR5-Inhibitor in der klinischen Entwicklung.
This compound ist einzigartig aufgrund seiner potenten In-vitro-Aktivität gegen ein breites Spektrum von HIV-Subtypen und seiner günstigen pharmakokinetischen und pharmakodynamischen Eigenschaften . Es hat sich in klinischen Studien als vielversprechend erwiesen, insbesondere bei vorbehandelten Patienten .
Eigenschaften
IUPAC Name |
(4,6-dimethylpyrimidin-5-yl)-[4-[(3S)-4-[(1R)-2-methoxy-1-[4-(trifluoromethyl)phenyl]ethyl]-3-methylpiperazin-1-yl]-4-methylpiperidin-1-yl]methanone | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C28H38F3N5O2/c1-19-16-35(14-15-36(19)24(17-38-5)22-6-8-23(9-7-22)28(29,30)31)27(4)10-12-34(13-11-27)26(37)25-20(2)32-18-33-21(25)3/h6-9,18-19,24H,10-17H2,1-5H3/t19-,24-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CNPVJJQCETWNEU-CYFREDJKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1CN(CCN1C(COC)C2=CC=C(C=C2)C(F)(F)F)C3(CCN(CC3)C(=O)C4=C(N=CN=C4C)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H]1CN(CCN1[C@@H](COC)C2=CC=C(C=C2)C(F)(F)F)C3(CCN(CC3)C(=O)C4=C(N=CN=C4C)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C28H38F3N5O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID40897719 | |
| Record name | Vicriviroc | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40897719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
533.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Vicriviroc is a once daily oral inhibitor of CCR5. It noncompetitively binds to a hydrophobic pocket between transmembrance helices by the extracellular side of CCR5. This allosteric antagonism causes a conformational change in the protein preventing binding of gp120 to CCR5. This prevents the entry of HIV into the cell. | |
| Record name | Vicriviroc | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06652 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
306296-47-9, 394730-30-4 | |
| Record name | Vicriviroc | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=306296-47-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Vicriviroc [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0306296479 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | SCH-D (Old RN) | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0394730304 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Vicriviroc | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06652 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Vicriviroc | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40897719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | VICRIVIROC | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/TL515DW4QS | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
A: Vicriviroc is a CCR5 antagonist, meaning it binds to the CCR5 receptor on the surface of CD4+ cells []. By doing so, it blocks the interaction between the HIV-1 envelope glycoprotein gp120 and CCR5, preventing viral entry into the cell [, , ].
A: The primary downstream effect is the inhibition of HIV-1 replication by preventing viral entry [, ]. Blocking CCR5 also inhibits the signaling cascade typically initiated by chemokine binding, potentially affecting immune cell migration and activation [, ].
A: The molecular formula of this compound is C27H39F3N6O2. Its molecular weight is 536.63 g/mol [].
A: Yes, studies have utilized various spectroscopic techniques to characterize this compound, including NMR spectroscopy and X-ray analysis. These studies have provided insights into the 3D properties and conformational behavior of the molecule, particularly concerning the arrangement at the planar amido function and the conformational preferences of the piperazine and piperidine rings [].
ANone: The provided research focuses primarily on this compound's biological activity and pharmacological properties. Therefore, detailed information regarding material compatibility and stability under various conditions is limited within these studies.
A: this compound acts as a receptor antagonist and does not exhibit catalytic properties []. Its primary mechanism involves competitive binding to the CCR5 receptor, inhibiting HIV-1 entry.
A: Yes, computational chemistry and modeling have been employed to understand the interactions of this compound with the CCR5 receptor []. Models have been developed to simulate the binding of this compound to the transmembrane domain of CCR5 and analyze its impact on receptor conformation and interaction with HIV-1 gp120 [].
A: Research has shown that even single amino acid substitutions in the V3 loop region of the HIV-1 envelope glycoprotein gp120 can significantly impact this compound resistance []. Specific mutations, such as K305R and K319T, have been linked to altered resistance and infectivity profiles [].
A: While specific stability data is limited within the provided research, it is known that this compound is metabolized primarily by the CYP3A4 enzyme system [, ]. Co-administration with CYP3A4 inhibitors or inducers may necessitate dose adjustments []. Formulation strategies, such as the development of intravaginal rings, have been explored to achieve sustained drug delivery and potentially improve bioavailability [].
ANone: The research primarily focuses on the scientific and clinical aspects of this compound. Detailed information regarding specific SHE regulations and compliance is not extensively discussed.
A: this compound exhibits good oral bioavailability and a long half-life, permitting once-daily dosing [, , , ]. It is primarily metabolized by the CYP3A4 enzyme system, necessitating dose adjustments when co-administered with CYP3A4 inhibitors or inducers [, , , ]. Studies in humans, monkeys, and rats reveal that the drug is metabolized through O-demethylation, N-dealkylation, oxidation, and glucuronidation [].
A: Studies have shown a correlation between higher this compound concentrations, particularly the minimum plasma concentration (Cmin), and a greater decrease in HIV-1 RNA levels []. Subjects with a Cmin above 54 ng/mL exhibited a significantly larger mean decrease in viral load compared to those with a lower Cmin []. This correlation suggests a concentration-dependent antiviral effect of this compound.
A: Yes, this compound has demonstrated potent antiviral activity in both in vitro and in vivo studies. In vitro, it effectively inhibits the replication of various HIV-1 isolates, including drug-resistant strains, and exhibits synergistic effects with other antiretroviral agents []. In clinical trials, this compound, when administered with a boosted protease inhibitor-containing regimen, significantly reduced HIV-1 RNA levels in treatment-experienced patients compared to placebo, with sustained efficacy observed over 48 weeks []. Furthermore, studies have explored its potential in treatment-naïve patients [, ].
A: Resistance to this compound primarily arises from mutations within the HIV-1 envelope glycoprotein, specifically the V3 loop region [, , , ]. These mutations can reduce the binding affinity of this compound to CCR5, thereby diminishing its antiviral activity.
A: Cross-resistance to other CCR5 antagonists, such as TAK-779, has been reported in HIV-1 isolates with reduced susceptibility to this compound []. This suggests that mutations conferring resistance to one CCR5 antagonist might confer resistance to others within the same class.
A: Research has investigated intravaginal rings containing this compound and MK-2048, a novel nucleoside reverse transcriptase translocation inhibitor, for sustained drug delivery to prevent HIV-1 infection []. This approach aims to achieve high drug concentrations in the vaginal mucosa while minimizing systemic exposure.
A: Liquid chromatography coupled with mass spectrometry (LC-MS) is a primary method used to identify and quantify this compound and its metabolites in biological samples []. This technique provides high sensitivity and selectivity for analyzing drug concentrations in various matrices.
ANone: The provided research focuses primarily on this compound's biological activity, pharmacology, and clinical effects. Therefore, information regarding environmental impact, dissolution/solubility, analytical method validation, alternatives, recycling/waste management, research infrastructure/resources, and related aspects is limited within these studies.
ANone: this compound emerged as part of the research effort to develop new antiretroviral therapies, specifically targeting the HIV-1 entry process. It represents a second-generation CCR5 antagonist, building upon earlier research on compounds like SCH-C. Despite its initial promise, this compound faced challenges in phase III clinical trials, leading to the discontinuation of its development as a treatment for HIV-1 infection. Nevertheless, the knowledge gained from this compound research has contributed to the understanding of HIV-1 entry mechanisms and the development of other antiretroviral drugs.
A: While the primary focus of this compound research has been on HIV-1 treatment, the knowledge gained has broader implications. For instance, understanding the role of CCR5 in viral entry and the mechanisms of CCR5 antagonist action can inform research on other viruses that utilize this receptor, such as some flaviviruses []. Additionally, this compound's effects on CCR5 signaling and immune cell migration could have implications for understanding and potentially treating inflammatory and autoimmune diseases.
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.


![Boc-[15N]Tyr-OH](/img/structure/B613742.png)

![4-Pentenoic acid, 2-[[(1,1-dimethylethoxy)carbonyl]amino]-2-methyl-, ethyl ester, (2R)-](/img/structure/B613751.png)