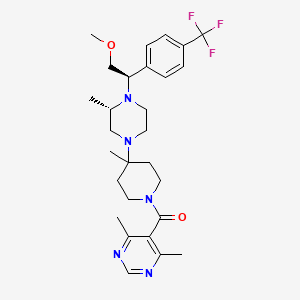
Vicriviroc
Descripción general
Descripción
Vicriviroc es un compuesto a base de pirimidina que funciona como un inhibidor de la entrada de CCR5 del VIH-1. Fue desarrollado por la empresa farmacéutica Schering-Plough y se ha investigado por su potencial para controlar las infecciones por VIH-1 . This compound inhibe la interacción del VIH-1 con el receptor CCR5, evitando que el virus ingrese a las células huésped .
Aplicaciones Científicas De Investigación
Mecanismo De Acción
Vicriviroc funciona como un antagonista alostérico no competitivo del receptor CCR5. Se une a un bolsillo hidrofóbico entre las hélices transmembrana cerca de la superficie extracelular del receptor CCR5. Esta unión provoca un cambio conformacional en el receptor, impidiendo la unión de la proteína gp120 del VIH-1 a CCR5. Como resultado, se inhibe la entrada del VIH-1 en las células huésped, bloqueando las etapas iniciales del ciclo de vida viral .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción
La síntesis de Vicriviroc implica múltiples pasos, comenzando con la preparación de intermedios claveLas condiciones de reacción específicas, como la temperatura, los disolventes y los catalizadores, se optimizan para garantizar un alto rendimiento y pureza .
Métodos de producción industrial
La producción industrial de this compound sigue rutas sintéticas similares, pero se amplía para satisfacer las demandas comerciales. El proceso implica estrictas medidas de control de calidad para garantizar la coherencia y el cumplimiento de las normas reglamentarias. Se pueden emplear técnicas avanzadas, como la química de flujo continuo y la síntesis automatizada, para mejorar la eficiencia y reducir los costes de producción .
Análisis De Reacciones Químicas
Tipos de reacciones
Vicriviroc experimenta diversas reacciones químicas, que incluyen:
Oxidación: This compound puede oxidarse para formar this compound N-óxido, un metabolito mayoritario.
Reducción: Las reacciones de reducción pueden utilizarse para modificar grupos funcionales específicos dentro de la molécula.
Sustitución: Las reacciones de sustitución pueden utilizarse para introducir o reemplazar sustituyentes en el núcleo de pirimidina.
Reactivos y condiciones comunes
Los reactivos comunes utilizados en estas reacciones incluyen agentes oxidantes como las enzimas citocromo P450 para la oxidación, agentes reductores para la reducción y nucleófilos para las reacciones de sustitución. Las condiciones de reacción, como la temperatura, el pH y la elección del disolvente, se controlan cuidadosamente para lograr los resultados deseados .
Productos principales formados
Los principales productos formados a partir de estas reacciones incluyen this compound N-óxido y otros metabolitos que resultan de la modificación del compuesto original .
Comparación Con Compuestos Similares
Vicriviroc forma parte de una clase de compuestos conocidos como inhibidores de CCR5. Compuestos similares incluyen:
Maraviroc: Otro inhibidor de CCR5 aprobado para su uso clínico en el tratamiento de las infecciones por VIH-1.
Aplaviroc: Un inhibidor de CCR5 que se descontinuó debido a preocupaciones por hepatotoxicidad.
INCB009471: Un inhibidor de CCR5 en desarrollo clínico.
TBR 652: Otro inhibidor de CCR5 en investigación.
This compound es único debido a su potente actividad in vitro contra una amplia gama de subtipos del VIH y sus propiedades farmacocinéticas y farmacodinámicas favorables . Ha mostrado promesas en ensayos clínicos, especialmente en pacientes con experiencia en el tratamiento .
Propiedades
IUPAC Name |
(4,6-dimethylpyrimidin-5-yl)-[4-[(3S)-4-[(1R)-2-methoxy-1-[4-(trifluoromethyl)phenyl]ethyl]-3-methylpiperazin-1-yl]-4-methylpiperidin-1-yl]methanone | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C28H38F3N5O2/c1-19-16-35(14-15-36(19)24(17-38-5)22-6-8-23(9-7-22)28(29,30)31)27(4)10-12-34(13-11-27)26(37)25-20(2)32-18-33-21(25)3/h6-9,18-19,24H,10-17H2,1-5H3/t19-,24-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CNPVJJQCETWNEU-CYFREDJKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1CN(CCN1C(COC)C2=CC=C(C=C2)C(F)(F)F)C3(CCN(CC3)C(=O)C4=C(N=CN=C4C)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H]1CN(CCN1[C@@H](COC)C2=CC=C(C=C2)C(F)(F)F)C3(CCN(CC3)C(=O)C4=C(N=CN=C4C)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C28H38F3N5O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID40897719 | |
| Record name | Vicriviroc | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40897719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
533.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Vicriviroc is a once daily oral inhibitor of CCR5. It noncompetitively binds to a hydrophobic pocket between transmembrance helices by the extracellular side of CCR5. This allosteric antagonism causes a conformational change in the protein preventing binding of gp120 to CCR5. This prevents the entry of HIV into the cell. | |
| Record name | Vicriviroc | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06652 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
306296-47-9, 394730-30-4 | |
| Record name | Vicriviroc | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=306296-47-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Vicriviroc [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0306296479 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | SCH-D (Old RN) | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0394730304 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Vicriviroc | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06652 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Vicriviroc | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40897719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | VICRIVIROC | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/TL515DW4QS | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
A: Vicriviroc is a CCR5 antagonist, meaning it binds to the CCR5 receptor on the surface of CD4+ cells []. By doing so, it blocks the interaction between the HIV-1 envelope glycoprotein gp120 and CCR5, preventing viral entry into the cell [, , ].
A: The primary downstream effect is the inhibition of HIV-1 replication by preventing viral entry [, ]. Blocking CCR5 also inhibits the signaling cascade typically initiated by chemokine binding, potentially affecting immune cell migration and activation [, ].
A: The molecular formula of this compound is C27H39F3N6O2. Its molecular weight is 536.63 g/mol [].
A: Yes, studies have utilized various spectroscopic techniques to characterize this compound, including NMR spectroscopy and X-ray analysis. These studies have provided insights into the 3D properties and conformational behavior of the molecule, particularly concerning the arrangement at the planar amido function and the conformational preferences of the piperazine and piperidine rings [].
ANone: The provided research focuses primarily on this compound's biological activity and pharmacological properties. Therefore, detailed information regarding material compatibility and stability under various conditions is limited within these studies.
A: this compound acts as a receptor antagonist and does not exhibit catalytic properties []. Its primary mechanism involves competitive binding to the CCR5 receptor, inhibiting HIV-1 entry.
A: Yes, computational chemistry and modeling have been employed to understand the interactions of this compound with the CCR5 receptor []. Models have been developed to simulate the binding of this compound to the transmembrane domain of CCR5 and analyze its impact on receptor conformation and interaction with HIV-1 gp120 [].
A: Research has shown that even single amino acid substitutions in the V3 loop region of the HIV-1 envelope glycoprotein gp120 can significantly impact this compound resistance []. Specific mutations, such as K305R and K319T, have been linked to altered resistance and infectivity profiles [].
A: While specific stability data is limited within the provided research, it is known that this compound is metabolized primarily by the CYP3A4 enzyme system [, ]. Co-administration with CYP3A4 inhibitors or inducers may necessitate dose adjustments []. Formulation strategies, such as the development of intravaginal rings, have been explored to achieve sustained drug delivery and potentially improve bioavailability [].
ANone: The research primarily focuses on the scientific and clinical aspects of this compound. Detailed information regarding specific SHE regulations and compliance is not extensively discussed.
A: this compound exhibits good oral bioavailability and a long half-life, permitting once-daily dosing [, , , ]. It is primarily metabolized by the CYP3A4 enzyme system, necessitating dose adjustments when co-administered with CYP3A4 inhibitors or inducers [, , , ]. Studies in humans, monkeys, and rats reveal that the drug is metabolized through O-demethylation, N-dealkylation, oxidation, and glucuronidation [].
A: Studies have shown a correlation between higher this compound concentrations, particularly the minimum plasma concentration (Cmin), and a greater decrease in HIV-1 RNA levels []. Subjects with a Cmin above 54 ng/mL exhibited a significantly larger mean decrease in viral load compared to those with a lower Cmin []. This correlation suggests a concentration-dependent antiviral effect of this compound.
A: Yes, this compound has demonstrated potent antiviral activity in both in vitro and in vivo studies. In vitro, it effectively inhibits the replication of various HIV-1 isolates, including drug-resistant strains, and exhibits synergistic effects with other antiretroviral agents []. In clinical trials, this compound, when administered with a boosted protease inhibitor-containing regimen, significantly reduced HIV-1 RNA levels in treatment-experienced patients compared to placebo, with sustained efficacy observed over 48 weeks []. Furthermore, studies have explored its potential in treatment-naïve patients [, ].
A: Resistance to this compound primarily arises from mutations within the HIV-1 envelope glycoprotein, specifically the V3 loop region [, , , ]. These mutations can reduce the binding affinity of this compound to CCR5, thereby diminishing its antiviral activity.
A: Cross-resistance to other CCR5 antagonists, such as TAK-779, has been reported in HIV-1 isolates with reduced susceptibility to this compound []. This suggests that mutations conferring resistance to one CCR5 antagonist might confer resistance to others within the same class.
A: Research has investigated intravaginal rings containing this compound and MK-2048, a novel nucleoside reverse transcriptase translocation inhibitor, for sustained drug delivery to prevent HIV-1 infection []. This approach aims to achieve high drug concentrations in the vaginal mucosa while minimizing systemic exposure.
A: Liquid chromatography coupled with mass spectrometry (LC-MS) is a primary method used to identify and quantify this compound and its metabolites in biological samples []. This technique provides high sensitivity and selectivity for analyzing drug concentrations in various matrices.
ANone: The provided research focuses primarily on this compound's biological activity, pharmacology, and clinical effects. Therefore, information regarding environmental impact, dissolution/solubility, analytical method validation, alternatives, recycling/waste management, research infrastructure/resources, and related aspects is limited within these studies.
ANone: this compound emerged as part of the research effort to develop new antiretroviral therapies, specifically targeting the HIV-1 entry process. It represents a second-generation CCR5 antagonist, building upon earlier research on compounds like SCH-C. Despite its initial promise, this compound faced challenges in phase III clinical trials, leading to the discontinuation of its development as a treatment for HIV-1 infection. Nevertheless, the knowledge gained from this compound research has contributed to the understanding of HIV-1 entry mechanisms and the development of other antiretroviral drugs.
A: While the primary focus of this compound research has been on HIV-1 treatment, the knowledge gained has broader implications. For instance, understanding the role of CCR5 in viral entry and the mechanisms of CCR5 antagonist action can inform research on other viruses that utilize this receptor, such as some flaviviruses []. Additionally, this compound's effects on CCR5 signaling and immune cell migration could have implications for understanding and potentially treating inflammatory and autoimmune diseases.
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![Boc-[15N]Tyr-OH](/img/structure/B613742.png)

![[2,6-Dimethyl-4-(3-[2-(Z-amino)-ethylcarbamoyl]-propoxy)-benzenesulfonyl]-Dap(Boc)-OMe](/img/structure/B613750.png)
![4-trans-[Bis(t-butyloxycarbonyl)-guanidino]cyclohexane carboxylic acid](/img/structure/B613755.png)