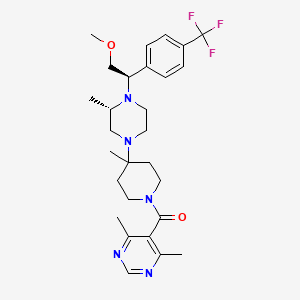
Vicriviroc
描述
维克瑞维洛克是一种嘧啶类化合物,作为 HIV-1 的 CCR5 进入抑制剂。 它由制药公司先灵葆雅开发,并已对其在管理 HIV-1 感染方面的潜力进行了研究 . 维克瑞维洛克抑制 HIV-1 与 CCR5 受体的相互作用,阻止病毒进入宿主细胞 .
作用机制
维克瑞维洛克作为 CCR5 受体的非竞争性变构拮抗剂。它与 CCR5 受体跨膜螺旋之间靠近细胞外表面的疏水口袋结合。这种结合会导致受体发生构象变化,阻止 HIV-1 gp120 蛋白与 CCR5 结合。 结果,HIV-1 进入宿主细胞被抑制,阻断病毒生命周期的初始阶段 .
准备方法
合成路线和反应条件
维克瑞维洛克的合成涉及多个步骤,从关键中间体的制备开始。 具体反应条件,如温度、溶剂和催化剂,经过优化以确保高产率和纯度 .
工业生产方法
维克瑞维洛克的工业生产遵循类似的合成路线,但已扩展规模以满足商业需求。该过程涉及严格的质量控制措施,以确保一致性和符合监管标准。 先进技术,例如连续流动化学和自动化合成,可能被用来提高效率并降低生产成本 .
化学反应分析
反应类型
维克瑞维洛克经历各种化学反应,包括:
氧化: 维克瑞维洛克可以被氧化形成维克瑞维洛克 N-氧化物,这是一种主要的代谢物.
还原: 还原反应可用于修饰分子中的特定官能团。
常用试剂和条件
这些反应中使用的常用试剂包括用于氧化的细胞色素 P450 酶等氧化剂、用于还原的还原剂和用于取代反应的亲核试剂。 反应条件,如温度、pH 和溶剂选择,经过仔细控制以实现预期的结果 .
形成的主要产品
科学研究应用
相似化合物的比较
维克瑞维洛克属于一类称为 CCR5 抑制剂的化合物。类似的化合物包括:
马拉维洛克: 另一种被批准用于临床治疗 HIV-1 感染的 CCR5 抑制剂.
阿普拉维洛克: 由于肝毒性问题而停止使用的一种 CCR5 抑制剂.
INCB009471: 正在进行临床开发的一种 CCR5 抑制剂.
TBR 652: 正在研究的另一种 CCR5 抑制剂.
维克瑞维洛克因其对多种 HIV 亚型的强大体外活性及其良好的药代动力学和药效学特性而具有独特性 . 它在临床试验中显示出希望,特别是在治疗经验丰富的患者中 .
属性
IUPAC Name |
(4,6-dimethylpyrimidin-5-yl)-[4-[(3S)-4-[(1R)-2-methoxy-1-[4-(trifluoromethyl)phenyl]ethyl]-3-methylpiperazin-1-yl]-4-methylpiperidin-1-yl]methanone | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C28H38F3N5O2/c1-19-16-35(14-15-36(19)24(17-38-5)22-6-8-23(9-7-22)28(29,30)31)27(4)10-12-34(13-11-27)26(37)25-20(2)32-18-33-21(25)3/h6-9,18-19,24H,10-17H2,1-5H3/t19-,24-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
CNPVJJQCETWNEU-CYFREDJKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1CN(CCN1C(COC)C2=CC=C(C=C2)C(F)(F)F)C3(CCN(CC3)C(=O)C4=C(N=CN=C4C)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H]1CN(CCN1[C@@H](COC)C2=CC=C(C=C2)C(F)(F)F)C3(CCN(CC3)C(=O)C4=C(N=CN=C4C)C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C28H38F3N5O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID40897719 | |
| Record name | Vicriviroc | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40897719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
533.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Vicriviroc is a once daily oral inhibitor of CCR5. It noncompetitively binds to a hydrophobic pocket between transmembrance helices by the extracellular side of CCR5. This allosteric antagonism causes a conformational change in the protein preventing binding of gp120 to CCR5. This prevents the entry of HIV into the cell. | |
| Record name | Vicriviroc | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06652 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
306296-47-9, 394730-30-4 | |
| Record name | Vicriviroc | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=306296-47-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Vicriviroc [INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0306296479 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | SCH-D (Old RN) | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0394730304 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Vicriviroc | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06652 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Vicriviroc | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40897719 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | VICRIVIROC | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/TL515DW4QS | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
A: Vicriviroc is a CCR5 antagonist, meaning it binds to the CCR5 receptor on the surface of CD4+ cells []. By doing so, it blocks the interaction between the HIV-1 envelope glycoprotein gp120 and CCR5, preventing viral entry into the cell [, , ].
A: The primary downstream effect is the inhibition of HIV-1 replication by preventing viral entry [, ]. Blocking CCR5 also inhibits the signaling cascade typically initiated by chemokine binding, potentially affecting immune cell migration and activation [, ].
A: The molecular formula of this compound is C27H39F3N6O2. Its molecular weight is 536.63 g/mol [].
A: Yes, studies have utilized various spectroscopic techniques to characterize this compound, including NMR spectroscopy and X-ray analysis. These studies have provided insights into the 3D properties and conformational behavior of the molecule, particularly concerning the arrangement at the planar amido function and the conformational preferences of the piperazine and piperidine rings [].
ANone: The provided research focuses primarily on this compound's biological activity and pharmacological properties. Therefore, detailed information regarding material compatibility and stability under various conditions is limited within these studies.
A: this compound acts as a receptor antagonist and does not exhibit catalytic properties []. Its primary mechanism involves competitive binding to the CCR5 receptor, inhibiting HIV-1 entry.
A: Yes, computational chemistry and modeling have been employed to understand the interactions of this compound with the CCR5 receptor []. Models have been developed to simulate the binding of this compound to the transmembrane domain of CCR5 and analyze its impact on receptor conformation and interaction with HIV-1 gp120 [].
A: Research has shown that even single amino acid substitutions in the V3 loop region of the HIV-1 envelope glycoprotein gp120 can significantly impact this compound resistance []. Specific mutations, such as K305R and K319T, have been linked to altered resistance and infectivity profiles [].
A: While specific stability data is limited within the provided research, it is known that this compound is metabolized primarily by the CYP3A4 enzyme system [, ]. Co-administration with CYP3A4 inhibitors or inducers may necessitate dose adjustments []. Formulation strategies, such as the development of intravaginal rings, have been explored to achieve sustained drug delivery and potentially improve bioavailability [].
ANone: The research primarily focuses on the scientific and clinical aspects of this compound. Detailed information regarding specific SHE regulations and compliance is not extensively discussed.
A: this compound exhibits good oral bioavailability and a long half-life, permitting once-daily dosing [, , , ]. It is primarily metabolized by the CYP3A4 enzyme system, necessitating dose adjustments when co-administered with CYP3A4 inhibitors or inducers [, , , ]. Studies in humans, monkeys, and rats reveal that the drug is metabolized through O-demethylation, N-dealkylation, oxidation, and glucuronidation [].
A: Studies have shown a correlation between higher this compound concentrations, particularly the minimum plasma concentration (Cmin), and a greater decrease in HIV-1 RNA levels []. Subjects with a Cmin above 54 ng/mL exhibited a significantly larger mean decrease in viral load compared to those with a lower Cmin []. This correlation suggests a concentration-dependent antiviral effect of this compound.
A: Yes, this compound has demonstrated potent antiviral activity in both in vitro and in vivo studies. In vitro, it effectively inhibits the replication of various HIV-1 isolates, including drug-resistant strains, and exhibits synergistic effects with other antiretroviral agents []. In clinical trials, this compound, when administered with a boosted protease inhibitor-containing regimen, significantly reduced HIV-1 RNA levels in treatment-experienced patients compared to placebo, with sustained efficacy observed over 48 weeks []. Furthermore, studies have explored its potential in treatment-naïve patients [, ].
A: Resistance to this compound primarily arises from mutations within the HIV-1 envelope glycoprotein, specifically the V3 loop region [, , , ]. These mutations can reduce the binding affinity of this compound to CCR5, thereby diminishing its antiviral activity.
A: Cross-resistance to other CCR5 antagonists, such as TAK-779, has been reported in HIV-1 isolates with reduced susceptibility to this compound []. This suggests that mutations conferring resistance to one CCR5 antagonist might confer resistance to others within the same class.
A: Research has investigated intravaginal rings containing this compound and MK-2048, a novel nucleoside reverse transcriptase translocation inhibitor, for sustained drug delivery to prevent HIV-1 infection []. This approach aims to achieve high drug concentrations in the vaginal mucosa while minimizing systemic exposure.
A: Liquid chromatography coupled with mass spectrometry (LC-MS) is a primary method used to identify and quantify this compound and its metabolites in biological samples []. This technique provides high sensitivity and selectivity for analyzing drug concentrations in various matrices.
ANone: The provided research focuses primarily on this compound's biological activity, pharmacology, and clinical effects. Therefore, information regarding environmental impact, dissolution/solubility, analytical method validation, alternatives, recycling/waste management, research infrastructure/resources, and related aspects is limited within these studies.
ANone: this compound emerged as part of the research effort to develop new antiretroviral therapies, specifically targeting the HIV-1 entry process. It represents a second-generation CCR5 antagonist, building upon earlier research on compounds like SCH-C. Despite its initial promise, this compound faced challenges in phase III clinical trials, leading to the discontinuation of its development as a treatment for HIV-1 infection. Nevertheless, the knowledge gained from this compound research has contributed to the understanding of HIV-1 entry mechanisms and the development of other antiretroviral drugs.
A: While the primary focus of this compound research has been on HIV-1 treatment, the knowledge gained has broader implications. For instance, understanding the role of CCR5 in viral entry and the mechanisms of CCR5 antagonist action can inform research on other viruses that utilize this receptor, such as some flaviviruses []. Additionally, this compound's effects on CCR5 signaling and immune cell migration could have implications for understanding and potentially treating inflammatory and autoimmune diseases.
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。



![Boc-[15N]Tyr-OH](/img/structure/B613742.png)
![[2,6-Dimethyl-4-(3-[2-(Z-amino)-ethylcarbamoyl]-propoxy)-benzenesulfonyl]-Dap(Boc)-OMe](/img/structure/B613750.png)
![4-[(Boc)2-guanidino]phenylacetic acid](/img/structure/B613756.png)