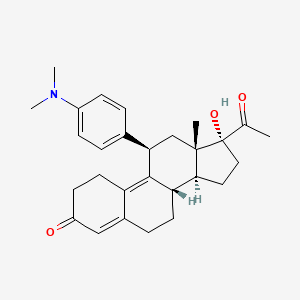
Acetato de ulipristal
Descripción general
Descripción
Ulipristal es un modulador selectivo del receptor de progesterona utilizado principalmente para la anticoncepción de emergencia y el tratamiento de los fibromas uterinos. Es un derivado de la 19-norprogesterona y exhibe tanto actividad antagonista como agonista parcial en el receptor de progesterona .
Aplicaciones Científicas De Investigación
Ulipristal tiene una amplia gama de aplicaciones de investigación científica:
Química: Utilizado como un compuesto modelo para estudiar los moduladores selectivos del receptor de progesterona.
Biología: Investigado por sus efectos en las vías celulares y la unión a receptores.
Medicina: Utilizado principalmente para la anticoncepción de emergencia y el tratamiento de los fibromas uterinos.
Industria: Utilizado en la industria farmacéutica para el desarrollo de píldoras anticonceptivas y tratamientos para fibromas .
Mecanismo De Acción
Ulipristal ejerce sus efectos uniéndose al receptor de progesterona, donde actúa como un antagonista y un agonista parcial. Esta unión inhibe la ovulación al prevenir la ruptura folicular y también puede afectar el endometrio para prevenir la implantación del embrión . Los objetivos moleculares incluyen el receptor de progesterona y, en menor medida, el receptor de glucocorticoides .
Análisis Bioquímico
Biochemical Properties
Ulipristal plays a significant role in biochemical reactions by interacting with various enzymes, proteins, and other biomolecules. It binds to the progesterone receptor, exhibiting both antagonistic and partial agonist activities. Additionally, ulipristal binds to the glucocorticoid receptor, although it has lower glucocorticoid activity compared to mifepristone . These interactions are crucial for its function as an emergency contraceptive and in the treatment of uterine fibroids.
Cellular Effects
Ulipristal affects various types of cells and cellular processes. In the treatment of uterine fibroids, ulipristal reduces the size of fibroids by inhibiting cell proliferation and inducing apoptosis . It also influences cell signaling pathways, gene expression, and cellular metabolism. For instance, ulipristal has been shown to delay or inhibit ovulation by affecting the endometrium, which may prevent embryo implantation .
Molecular Mechanism
The molecular mechanism of ulipristal involves its binding to the progesterone receptor, where it acts as both an antagonist and partial agonist . This binding prevents progestin from binding to the receptor, thereby inhibiting or delaying ovulation . Ulipristal also alters the normal endometrium, impairing implantation . Additionally, it inhibits the translocation of the glucocorticoid receptor to the nucleus, affecting gene expression .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of ulipristal change over time. Ulipristal is rapidly absorbed, with a peak plasma concentration occurring approximately one hour after ingestion . Its stability and degradation over time have been studied, showing that it can inhibit follicular rupture and induce early endometrial bleeding in a dose-dependent manner . Long-term effects on cellular function have also been observed, including changes in endometrial maturation and progesterone-dependent markers of implantation .
Dosage Effects in Animal Models
The effects of ulipristal vary with different dosages in animal models. A single post-ovulatory dose of ulipristal has been shown to impair post-fertilization events in mice, reducing the number of conceptuses and early implantation sites . High doses of ulipristal can cause histological and functional alterations in the uterine horns, affecting embryo-uterine interaction .
Metabolic Pathways
Ulipristal is metabolized primarily by the enzyme CYP3A4 and to a lesser extent by CYP1A2 . It is converted into mono-demethylated (active) and di-methylated (inactive) metabolites . These metabolic pathways are crucial for its pharmacokinetics and overall efficacy.
Transport and Distribution
Ulipristal is transported and distributed within cells and tissues, targeting the uterus, cervix, ovaries, and hypothalamus . It binds to plasma proteins with a high affinity, which influences its distribution and localization within the body . The transporters and binding proteins involved in its distribution play a significant role in its therapeutic effects.
Subcellular Localization
The subcellular localization of ulipristal involves its interaction with the glucocorticoid receptor, where it inhibits the receptor’s translocation to the nucleus . This inhibition affects the receptor’s activity and function, leading to changes in gene expression and cellular responses. Ulipristal’s targeting signals and post-translational modifications direct it to specific compartments or organelles, influencing its overall activity.
Métodos De Preparación
Rutas Sintéticas y Condiciones de Reacción
La síntesis de ulipristal implica múltiples pasos, comenzando con la 19-norprogesteronaLas condiciones de reacción típicamente implican el uso de reactivos como metil litio o reactivo de Grignard de metilo para las reacciones de adición, seguido de pasos de hidrólisis y cristalización para purificar el producto final .
Métodos de Producción Industrial
La producción industrial de ulipristal implica la optimización de la ruta sintética para garantizar un alto rendimiento y pureza. Esto incluye controlar las condiciones de hidrólisis, como la acidez, la temperatura y el tiempo de reacción, para obtener el producto deseado. El producto final se purifica utilizando solventes como etanol e isopropanol .
Análisis De Reacciones Químicas
Tipos de Reacciones
Ulipristal experimenta varias reacciones químicas, que incluyen:
Oxidación: Ulipristal puede oxidarse para formar diferentes metabolitos.
Reducción: Las reacciones de reducción pueden modificar los grupos cetónicos presentes en la molécula.
Sustitución: Las reacciones de sustitución pueden ocurrir en el grupo dimetilamino o en el grupo acetoxi.
Reactivos y Condiciones Comunes
Los reactivos comunes utilizados en estas reacciones incluyen agentes oxidantes como el permanganato de potasio y agentes reductores como el borohidruro de sodio. Las reacciones se llevan a cabo típicamente bajo condiciones controladas de temperatura y pH para garantizar la formación del producto deseado .
Principales Productos Formados
Los principales productos formados a partir de estas reacciones incluyen varios metabolitos que retienen la estructura central de ulipristal pero con modificaciones en grupos funcionales específicos .
Comparación Con Compuestos Similares
Compuestos Similares
Mifepristona: Otro antagonista del receptor de progesterona utilizado para el aborto médico y la anticoncepción de emergencia.
Levonorgestrel: Un progestágeno sintético utilizado en la anticoncepción de emergencia y los anticonceptivos hormonales.
Singularidad de Ulipristal
Ulipristal es único en su actividad dual como antagonista y agonista parcial en el receptor de progesterona, lo que le proporciona un perfil de eficacia y efectos secundarios distinto en comparación con otros compuestos como la mifepristona y el levonorgestrel .
Propiedades
IUPAC Name |
(8S,11R,13S,14S,17R)-17-acetyl-11-[4-(dimethylamino)phenyl]-17-hydroxy-13-methyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C28H35NO3/c1-17(30)28(32)14-13-25-23-11-7-19-15-21(31)10-12-22(19)26(23)24(16-27(25,28)2)18-5-8-20(9-6-18)29(3)4/h5-6,8-9,15,23-25,32H,7,10-14,16H2,1-4H3/t23-,24+,25-,27-,28-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HKDLNTKNLJPAIY-WKWWZUSTSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(=O)C1(CCC2C1(CC(C3=C4CCC(=O)C=C4CCC23)C5=CC=C(C=C5)N(C)C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(=O)[C@]1(CC[C@@H]2[C@@]1(C[C@@H](C3=C4CCC(=O)C=C4CC[C@@H]23)C5=CC=C(C=C5)N(C)C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C28H35NO3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID501025842 | |
| Record name | Ulipristal | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID501025842 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
433.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
The exact mechanism of action of ulipristal has been heavily debated. On one hand, the majority of official prescribing information labels, monographs, and prior research studies for ulipristal indicated as an emergency contraceptive suggest that its primary mechanism of action revolves around inhibiting or delaying ovulation by suppressing surges in LH that result in the postponement of follicular rupture. Conversely, some of the latest investigations pertaining to ulipristal's mechanism of action as an emergency contraceptive propose that it principally elicits its action by preventing embryo implantation, as opposed to preventing ovulation. Although previous investigations have shown that ulipristal essentially has the ability to prevent ovulation equivalent to placebo (ie. null effect or ability) when administered during LH peaks one to two days before ovulation, the agent still demonstrates a stable and consistently high contraceptive effect of approximately >=80% when used at this time. Subsequently, current studies attempt to investigate how ulipristal could elicit emergency contraception via ovulation prevention under circumstances where ovulation had already clearly been observed. Endometrial biopsy samples studied from such circumstances in such investigations subsequently show that the administered ulipristal causes endometrial tissue to become inhospitable and unsuitable for embryo implantation where a variety of genes characteristic of receptive, pro-gestational endometrium are downregulated. Nevertheless, most if not all proposed mechanisms commonly agree that ulipristal ultimately demonstrates its pharmacological effects by binding to human progesterone receptors and prevents natural, endogenous progesterone from occupying such receptors. Regardless, however, considering current and on-going research into ulipristal's ability to prevent embryo implantation, the notion that the medication can elicit post-fertilization effects potentially raises alerts and/or ethical debates over the use of ulipristal owing to potential abortifacient activity, which is considered to be on par or equipotent to that of mifepristone. Attention should be drawn to the fact that some prescribing information, however, such as the US FDA label for ulipristal indicated for emergency contraception, has included new supplementary commentary since 2018 that directly warns about ulipristal not being indicated for termination of existing pregnancies and suggesting that ulipristal use may confer alterations to the endometrium that may affect implantation and contribute to efficacy. In the treatment of fibroids, ulipristal has been shown to exert direct actions on fibroids reducing their size through inhibition of cell proliferation and induction of apoptosis. | |
| Record name | Ulipristal | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08867 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
159811-51-5 | |
| Record name | Ulipristal [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0159811515 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ulipristal | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB08867 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Ulipristal | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID501025842 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (8S,11R,13S,14S,17R)-17-acetyl-11-[4-(dimethylamino)phenyl]-17-hydroxy-13-methyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-3-one | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ULIPRISTAL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/6J5J15Q2X8 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.


![N-[(3S)-1-({6-chloro-3-[1-(4-chlorobenzyl)-4-phenyl-1H-imidazol-5-yl]-1H-indol-2-yl}carbonyl)pyrrolidin-3-yl]-N,N',N'-trimethylpropane-1,3-diamine](/img/structure/B1683313.png)
![5-(2,2-Diphenylacetyl)-4-[(4-methoxy-3-methylphenyl)methyl]-1,4,6,7-tetrahydroimidazo[4,5-c]pyridine-6-carboxylic acid](/img/structure/B1683315.png)