Éthosuximide
Vue d'ensemble
Description
L’éthosuximide est un médicament principalement utilisé pour traiter les absences, un type d’épilepsie. Il appartient à la classe des succinimides anticonvulsivants et est connu pour son efficacité dans la gestion des crises sans provoquer d’hépat toxicité significative . L’éthosuximide est administré par voie orale et est généralement bien toléré, les effets secondaires courants étant la perte d’appétit, les douleurs abdominales et la fatigue .
Mécanisme D'action
L’éthosuximide exerce ses effets en bloquant les canaux calciques dépendants du voltage de type T dans le cerveau . Ces canaux jouent un rôle crucial dans la génération de l’activité électrique rythmique des neurones. En inhibant ces canaux, l’éthosuximide réduit la survenue de décharges électriques anormales qui provoquent des crises . La cible moléculaire principale de l’éthosuximide est la sous-unité alpha-1G du canal calcique de type T .
Applications De Recherche Scientifique
Ethosuximide has a wide range of scientific research applications:
Analyse Biochimique
Biochemical Properties
Ethosuximide exerts its effects by interacting with T-type voltage-sensitive calcium channels . These channels mediate the entry of calcium ions into excitable cells and are involved in various calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division, and cell death .
Cellular Effects
Ethosuximide influences cell function by suppressing the paroxysmal three-cycle per second spike and wave activity associated with lapses of consciousness, which is common in absence (petit mal) seizures . The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the central nervous system to convulsive stimuli .
Molecular Mechanism
The molecular mechanism of Ethosuximide involves the antagonism of the postsynaptic T-type voltage-gated calcium channel . This action reduces the low threshold T-type calcium currents in thalamic neurons, which are involved in the spontaneous pacemaker oscillatory activity of thalamocortical circuitry .
Temporal Effects in Laboratory Settings
It is known that Ethosuximide is rapidly absorbed with a bioavailability of >90% . Its volume of distribution is 0.7 L/kg, and plasma protein binding is 0% .
Dosage Effects in Animal Models
In animal models, Ethosuximide has shown powerful analgesic effects through the blockade of T-type voltage-gated calcium currents in sensory neurons
Metabolic Pathways
Approximately 80% of Ethosuximide undergoes hepatic metabolism, mediated primarily by cytochrome P450 isoenzymes, with a major contribution from CYP3A and, to a lesser extent, from CYP2E and CYP2C/B . The remainder, around 20% of an administered dose of Ethosuximide, is excreted unchanged in the urine .
Transport and Distribution
After oral ingestion, Ethosuximide is rapidly absorbed with a Tmax of 1–4 hours . Its volume of distribution is 0.7 L/kg, indicating that it is distributed throughout the body .
Méthodes De Préparation
L’éthosuximide peut être synthétisé par différentes méthodes. Une voie de synthèse courante implique la réaction de l’acétoacétate d’éthyle avec la méthylamine pour former la 3-éthyl-3-méthyl-2,5-pyrrolidinedione . Les conditions réactionnelles impliquent généralement le chauffage des réactifs sous reflux en présence d’un solvant approprié. Les méthodes de production industrielles impliquent souvent des voies de synthèse similaires, mais à plus grande échelle, avec des étapes de purification supplémentaires pour garantir que le produit final répond aux normes pharmaceutiques .
Analyse Des Réactions Chimiques
L’éthosuximide subit plusieurs types de réactions chimiques, notamment :
Réduction : Bien que les réactions de réduction soient moins fréquentes, l’éthosuximide peut subir une réduction dans des conditions spécifiques pour produire différents produits.
Substitution : L’éthosuximide peut participer à des réactions de substitution, en particulier au niveau de l’atome d’azote du cycle succinimide.
Les réactifs couramment utilisés dans ces réactions comprennent les agents oxydants comme le peroxyde d’hydrogène et les agents réducteurs comme le borohydrure de sodium. Les principaux produits formés à partir de ces réactions dépendent des conditions spécifiques et des réactifs utilisés .
Applications de la recherche scientifique
L’éthosuximide a un large éventail d’applications de recherche scientifique :
Comparaison Avec Des Composés Similaires
L’éthosuximide fait partie de la famille des succinimides anticonvulsivants, qui comprend également la méthsuximide et la phensuximide . Comparé à ces composés, l’éthosuximide est préféré pour le traitement des absences en raison de son risque plus faible d’hépat toxicité et de son meilleur profil d’effets secondaires . D’autres composés similaires comprennent l’acide valproïque et la lévétiracétam, qui sont également utilisés pour gérer les crises mais ont des mécanismes d’action et des profils d’effets secondaires différents .
Composés similaires
- Méthsuximide
- Phensuximide
- Acide valproïque
- Lévétiracétam
La capacité unique de l’éthosuximide à bloquer sélectivement les canaux calciques de type T le rend particulièrement efficace pour les absences, le distinguant des autres anticonvulsivants .
Propriétés
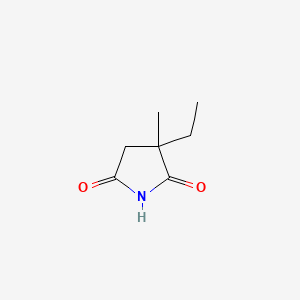
IUPAC Name |
3-ethyl-3-methylpyrrolidine-2,5-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C7H11NO2/c1-3-7(2)4-5(9)8-6(7)10/h3-4H2,1-2H3,(H,8,9,10) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HAPOVYFOVVWLRS-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC1(CC(=O)NC1=O)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C7H11NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7023019 | |
| Record name | Ethosuximide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023019 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
141.17 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Ethosuximide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014731 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Freely soluble in water, VERY SLIGHTLY SOL IN SOLVENT HEXANE, 1.01e+02 g/L | |
| Record name | Ethosuximide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00593 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ETHOSUXIMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1119 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ethosuximide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014731 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Binds to T-type voltage sensitive calcium channels. Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1G gives rise to T-type calcium currents. T-type calcium channels belong to the "low-voltage activated (LVA)" group and are strongly blocked by mibefradil. A particularity of this type of channels is an opening at quite negative potentials and a voltage-dependent inactivation. T-type channels serve pacemaking functions in both central neurons and cardiac nodal cells and support calcium signaling in secretory cells and vascular smooth muscle. They may also be involved in the modulation of firing patterns of neurons which is important for information processing as well as in cell growth processes., Succinimide anticonvulsants are thought to increase the seizure threshold and suppress the paroxysmal three-cycle-per-second spike-and-wave pattern seen with absence (petit mal) seizures. The frequency of attacks is reduced by depression of nerve transmission in the motor cortex. These effects may be due to direct modification of membrane function in excitable cells and/or alteration of chemically mediated neurotransmission. The specific effect of ethosuximide against absence seizures appears to be due to its ability to block T-type calcium channels at concentrations that do not affect other ion channels. /Succinimide Anticonvulsants/, Ethosuximide reduces low threshold Ca(2+) currents (T currents) in thalamic neurons. The thalamus plays an important role in generation of 3 Hz spike-wave rhythms typical of absence seizures. Neurons in the thalamus exhibit a large amplitude T current spike that underlies bursts of action potentials and likely plays an important role in thalamic oscillatory activity such as 3 Hz spike-wave activity. At clinically relevant concentrations, ethosuximide inhibits the T current, as evident in voltage-clamp recordings in acutely isolated, ventrobasal thalamic neurons from rats and guinea pigs. Ethosuximide reduces this current without modifying the voltage dependence of steady-state inactivation or the time course of recovery from inactivation. By contrast, succinimide derivatives with convulsant properties do not inhibit this current. Ethosuximide does not inhibit sustained repetitive firing or enhance GABA responses at clinically relevant concentrations. Current data are consistent with the idea that inhibition of T currents is the mechanism by which ethosuximide inhibits absence seizures., Ethosuximide is an alternative medicament that is used for coupling of petit mal, especially in childhood. Some clinical observations show that it has secondary effects on the gastro intestinal tract (GIT). The present research tries to define the characteristics of Ethosuximide--the inducted secondary effects on the GIT, and to explain some of the possible mechanisms that cause them. The changes that occur in the GIT of patients cured with Ethosuximide are registered roentgenologically. The main change is the atony of the stomach and intestines and the reduced peristaltic activity. The influence of Ethosuximide is examined on smooth muscular samples of human stomach, taken in vitro using stomach resection. The medicament authoritatively reduce the spontaneous bioelectrical activity of the smooth muscular tissue, influences mainly it's components that have Ca+ nature. Together with that is indicated relaxation of the smooth muscular samples. In that research is expressed the thesis that this Ethosuximide reduction of the Ca(+)-influx in the smooth muscular cells and the related relaxation probably are one of the main reasons of the secondary effects on the GIT., Ethosuximide is one of the means of treatment of minor epilepsy but hardly any data on its mechanism of action are available in the literature. Anticonvulsant agents are known to bring about changes in the functions and in the interaction between some of the mediator systems within the central nervous system. An assessment of the status of neuromediator systems can be made on the basis of the response of isolated smooth muscle strips to the action of agonists and antagonists of various receptors. It was found by the pharmacological analysis of isolated strips from the rat stomach (antrum and corpus strips), the seminal duct and the cervical vein that ethosuximide induces a reduction in the physical contractile activity and the tone of smooth muscle preparations. Smooth muscle relaxation caused by ethosuximide is not blocked by different receptor inhibitors such as dihydroergotamine, propranolol, atropine, chlorpromazine, haloperidol and indomethacin. Ethosuximide causes a significant reduction in the physical contraction of smooth muscles produced by potassium chloride depolarization, with a stronger impact on the subsequent tonic contraction caused by calcium ions. A reduction in the potassium content of the solution has no effect on the nature of the action of ethosutimide. It is thus assumed that the probable mechanism of action of ethosuximide consists in lowering calcium transport since the inhibitors of calcium transport sodium nitroprusside and verapamil intensify the blocking effect of ethosuximide on smooth muscle contractile activity. | |
| Record name | Ethosuximide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00593 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ETHOSUXIMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1119 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from acetone and ether, WHITE TO OFF-WHITE CRYSTALLINE POWDER OR WAXY SOLID | |
CAS No. |
77-67-8 | |
| Record name | Ethosuximide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=77-67-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ethosuximide [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000077678 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ethosuximide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00593 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ethosuximide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758192 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | ethosuximide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=64013 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Ethosuximide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023019 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Ethosuximide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.954 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ETHOSUXIMIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/5SEH9X1D1D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | ETHOSUXIMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1119 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ethosuximide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014731 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
64 - 65 °C, 64.5 °C | |
| Record name | Ethosuximide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00593 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ETHOSUXIMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1119 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ethosuximide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014731 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details















Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.