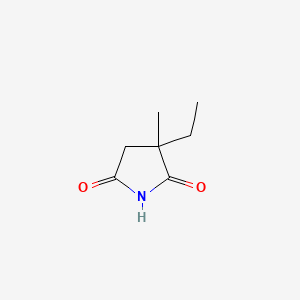
エトスクシミド
概要
説明
エトスクシミドは、主にてんかんの一種である失神発作の治療に使用される薬剤です。 これは、サクシニミド系抗てんかん薬に属し、有意な肝毒性を引き起こすことなく、発作の管理に有効であることで知られています 。 エトスクシミドは経口摂取され、一般的に忍容性が高く、一般的な副作用には食欲不振、腹痛、疲労などがあります .
製法
エトスクシミドは、さまざまな方法で合成することができます。 一般的な合成経路の1つは、アセト酢酸エチルとメチルアミンを反応させて、3-エチル-3-メチル-2,5-ピロリジンジオンを生成することです 。 反応条件としては、通常、適切な溶媒の存在下で、反応物を還流下で加熱することが含まれます。 工業生産方法では、多くの場合、同様の合成経路が採用されますが、より大規模に行われ、最終生成物が医薬品基準を満たすように、追加の精製工程が行われます .
作用機序
エトスクシミドは、脳内のT型電圧依存性カルシウムチャネルを遮断することで効果を発揮します 。 これらのチャネルは、ニューロンのリズミカルな電気的活動の発生に重要な役割を果たしています。 これらのチャネルを阻害することにより、エトスクシミドは、発作を引き起こす異常な電気的放電の発生を抑制します 。 エトスクシミドの主要な分子標的は、T型カルシウムチャネルのアルファ1Gサブユニットです .
科学的研究の応用
Ethosuximide has a wide range of scientific research applications:
生化学分析
Biochemical Properties
Ethosuximide exerts its effects by interacting with T-type voltage-sensitive calcium channels . These channels mediate the entry of calcium ions into excitable cells and are involved in various calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division, and cell death .
Cellular Effects
Ethosuximide influences cell function by suppressing the paroxysmal three-cycle per second spike and wave activity associated with lapses of consciousness, which is common in absence (petit mal) seizures . The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the central nervous system to convulsive stimuli .
Molecular Mechanism
The molecular mechanism of Ethosuximide involves the antagonism of the postsynaptic T-type voltage-gated calcium channel . This action reduces the low threshold T-type calcium currents in thalamic neurons, which are involved in the spontaneous pacemaker oscillatory activity of thalamocortical circuitry .
Temporal Effects in Laboratory Settings
It is known that Ethosuximide is rapidly absorbed with a bioavailability of >90% . Its volume of distribution is 0.7 L/kg, and plasma protein binding is 0% .
Dosage Effects in Animal Models
In animal models, Ethosuximide has shown powerful analgesic effects through the blockade of T-type voltage-gated calcium currents in sensory neurons
Metabolic Pathways
Approximately 80% of Ethosuximide undergoes hepatic metabolism, mediated primarily by cytochrome P450 isoenzymes, with a major contribution from CYP3A and, to a lesser extent, from CYP2E and CYP2C/B . The remainder, around 20% of an administered dose of Ethosuximide, is excreted unchanged in the urine .
Transport and Distribution
After oral ingestion, Ethosuximide is rapidly absorbed with a Tmax of 1–4 hours . Its volume of distribution is 0.7 L/kg, indicating that it is distributed throughout the body .
準備方法
Ethosuximide can be synthesized through various methods. One common synthetic route involves the reaction of ethyl acetoacetate with methylamine to form 3-ethyl-3-methyl-2,5-pyrrolidinedione . The reaction conditions typically include heating the reactants under reflux in the presence of a suitable solvent. Industrial production methods often involve similar synthetic routes but on a larger scale, with additional purification steps to ensure the final product meets pharmaceutical standards .
化学反応の分析
エトスクシミドは、次のようないくつかのタイプの化学反応を受けます。
酸化: エトスクシミドは、主に肝臓におけるシトクロムP450酵素の作用により、さまざまな代謝産物に変換されるように酸化される可能性があります.
還元: 還元反応はあまり一般的ではありませんが、エトスクシミドは特定の条件下で還元され、異なる生成物を生じる可能性があります。
これらの反応で使用される一般的な試薬には、過酸化水素などの酸化剤と、水素化ホウ素ナトリウムなどの還元剤が含まれます。 これらの反応から生成される主要な生成物は、使用される特定の条件と試薬によって異なります .
科学研究への応用
エトスクシミドは、幅広い科学研究への応用が期待されています。
類似化合物との比較
エトスクシミドは、メトスクシミドやフェンスキシミドなど、サクシニミド系抗てんかん薬のファミリーに属しています 。 これらの化合物と比較して、エトスクシミドは、肝毒性のリスクが低く、副作用のプロフィールが良好であるため、失神発作の治療に好まれています 。 その他の類似化合物には、バルプロ酸やレベチラセタムなどがあり、これらは発作の管理にも使用されますが、作用機序や副作用のプロフィールが異なります .
類似化合物
- メトスクシミド
- フェンスキシミド
- バルプロ酸
- レベチラセタム
エトスクシミドは、T型カルシウムチャネルを選択的に遮断するという独特の能力を持つため、特に失神発作に有効であり、他の抗てんかん薬とは一線を画しています .
特性
IUPAC Name |
3-ethyl-3-methylpyrrolidine-2,5-dione | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C7H11NO2/c1-3-7(2)4-5(9)8-6(7)10/h3-4H2,1-2H3,(H,8,9,10) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HAPOVYFOVVWLRS-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC1(CC(=O)NC1=O)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C7H11NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7023019 | |
| Record name | Ethosuximide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023019 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
141.17 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Ethosuximide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014731 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Freely soluble in water, VERY SLIGHTLY SOL IN SOLVENT HEXANE, 1.01e+02 g/L | |
| Record name | Ethosuximide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00593 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ETHOSUXIMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1119 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ethosuximide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014731 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Binds to T-type voltage sensitive calcium channels. Voltage-sensitive calcium channels (VSCC) mediate the entry of calcium ions into excitable cells and are also involved in a variety of calcium-dependent processes, including muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division and cell death. The isoform alpha-1G gives rise to T-type calcium currents. T-type calcium channels belong to the "low-voltage activated (LVA)" group and are strongly blocked by mibefradil. A particularity of this type of channels is an opening at quite negative potentials and a voltage-dependent inactivation. T-type channels serve pacemaking functions in both central neurons and cardiac nodal cells and support calcium signaling in secretory cells and vascular smooth muscle. They may also be involved in the modulation of firing patterns of neurons which is important for information processing as well as in cell growth processes., Succinimide anticonvulsants are thought to increase the seizure threshold and suppress the paroxysmal three-cycle-per-second spike-and-wave pattern seen with absence (petit mal) seizures. The frequency of attacks is reduced by depression of nerve transmission in the motor cortex. These effects may be due to direct modification of membrane function in excitable cells and/or alteration of chemically mediated neurotransmission. The specific effect of ethosuximide against absence seizures appears to be due to its ability to block T-type calcium channels at concentrations that do not affect other ion channels. /Succinimide Anticonvulsants/, Ethosuximide reduces low threshold Ca(2+) currents (T currents) in thalamic neurons. The thalamus plays an important role in generation of 3 Hz spike-wave rhythms typical of absence seizures. Neurons in the thalamus exhibit a large amplitude T current spike that underlies bursts of action potentials and likely plays an important role in thalamic oscillatory activity such as 3 Hz spike-wave activity. At clinically relevant concentrations, ethosuximide inhibits the T current, as evident in voltage-clamp recordings in acutely isolated, ventrobasal thalamic neurons from rats and guinea pigs. Ethosuximide reduces this current without modifying the voltage dependence of steady-state inactivation or the time course of recovery from inactivation. By contrast, succinimide derivatives with convulsant properties do not inhibit this current. Ethosuximide does not inhibit sustained repetitive firing or enhance GABA responses at clinically relevant concentrations. Current data are consistent with the idea that inhibition of T currents is the mechanism by which ethosuximide inhibits absence seizures., Ethosuximide is an alternative medicament that is used for coupling of petit mal, especially in childhood. Some clinical observations show that it has secondary effects on the gastro intestinal tract (GIT). The present research tries to define the characteristics of Ethosuximide--the inducted secondary effects on the GIT, and to explain some of the possible mechanisms that cause them. The changes that occur in the GIT of patients cured with Ethosuximide are registered roentgenologically. The main change is the atony of the stomach and intestines and the reduced peristaltic activity. The influence of Ethosuximide is examined on smooth muscular samples of human stomach, taken in vitro using stomach resection. The medicament authoritatively reduce the spontaneous bioelectrical activity of the smooth muscular tissue, influences mainly it's components that have Ca+ nature. Together with that is indicated relaxation of the smooth muscular samples. In that research is expressed the thesis that this Ethosuximide reduction of the Ca(+)-influx in the smooth muscular cells and the related relaxation probably are one of the main reasons of the secondary effects on the GIT., Ethosuximide is one of the means of treatment of minor epilepsy but hardly any data on its mechanism of action are available in the literature. Anticonvulsant agents are known to bring about changes in the functions and in the interaction between some of the mediator systems within the central nervous system. An assessment of the status of neuromediator systems can be made on the basis of the response of isolated smooth muscle strips to the action of agonists and antagonists of various receptors. It was found by the pharmacological analysis of isolated strips from the rat stomach (antrum and corpus strips), the seminal duct and the cervical vein that ethosuximide induces a reduction in the physical contractile activity and the tone of smooth muscle preparations. Smooth muscle relaxation caused by ethosuximide is not blocked by different receptor inhibitors such as dihydroergotamine, propranolol, atropine, chlorpromazine, haloperidol and indomethacin. Ethosuximide causes a significant reduction in the physical contraction of smooth muscles produced by potassium chloride depolarization, with a stronger impact on the subsequent tonic contraction caused by calcium ions. A reduction in the potassium content of the solution has no effect on the nature of the action of ethosutimide. It is thus assumed that the probable mechanism of action of ethosuximide consists in lowering calcium transport since the inhibitors of calcium transport sodium nitroprusside and verapamil intensify the blocking effect of ethosuximide on smooth muscle contractile activity. | |
| Record name | Ethosuximide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00593 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ETHOSUXIMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1119 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from acetone and ether, WHITE TO OFF-WHITE CRYSTALLINE POWDER OR WAXY SOLID | |
CAS No. |
77-67-8 | |
| Record name | Ethosuximide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=77-67-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Ethosuximide [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000077678 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Ethosuximide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00593 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ethosuximide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758192 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | ethosuximide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=64013 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Ethosuximide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7023019 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Ethosuximide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.954 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ETHOSUXIMIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/5SEH9X1D1D | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | ETHOSUXIMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1119 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ethosuximide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014731 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
64 - 65 °C, 64.5 °C | |
| Record name | Ethosuximide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00593 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | ETHOSUXIMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1119 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Ethosuximide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014731 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details















Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。