Maraviroc
Vue d'ensemble
Description
Maraviroc est un antagoniste du récepteur des chimiokines développé par Pfizer, commercialisé sous les noms de marque Selzentry aux États-Unis et Celsentri dans l'Union européenne . Il est principalement utilisé comme médicament antirétroviral pour le traitement de l'infection à VIH-1 tropique pour le CCR5 . This compound agit en bloquant le récepteur CCR5 à la surface de certaines cellules humaines, empêchant le virus du VIH de pénétrer dans ces cellules .
Mécanisme D'action
Target of Action
Maraviroc, developed by Pfizer, is a chemokine receptor antagonist drug . Its primary target is the human chemokine receptor CCR5 . This receptor is present on the membrane of CD4 cells (T-cells), which are a type of white blood cell that plays a key role in the immune system .
Mode of Action
This compound works by blocking HIV from entering human cells . Specifically, it is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120 . By binding to CCR5, this compound prevents the interaction of HIV-1 gp120 with CCR5-tropic HIV-1, thereby inhibiting the virus from entering the cell .
Biochemical Pathways
This compound’s action affects the HIV entry process . The drug interferes with the interaction between the HIV-1 gp120 protein and the CCR5 receptor, which is an essential step for the virus to enter the host cell . By blocking this interaction, this compound prevents the virus from fusing with the human cell membrane .
Pharmacokinetics
This compound is extensively metabolized by CYP3A4 , with renal clearance accounting for approximately 23% of total clearance . The half-life of this compound is approximately 16 hours .
Result of Action
The molecular and cellular effects of this compound’s action result in the inhibition of HIV-1 entry into human cells . By preventing the virus from entering the cells, this compound can effectively reduce the progression of HIV-1 infection .
Action Environment
The efficacy and stability of this compound can be influenced by various environmental factors. For instance, the sensitivity of tropism testing can impact the treatment outcome of this compound-containing regimens . Additionally, this compound’s exposure is altered by agents that modulate the activity of CYP3A4, and in some circumstances, this compound dose adjustment is necessary .
Applications De Recherche Scientifique
Maraviroc a une large gamme d'applications en recherche scientifique:
Médecine: Cliniquement, this compound est utilisé en association avec d'autres agents antirétroviraux pour traiter l'infection à VIH-1.
Mécanisme d'action
This compound fonctionne comme un inhibiteur d'entrée en se liant sélectivement au récepteur CCR5 à la surface des cellules humaines . Cette liaison empêche la protéine gp120 du VIH-1 de s'associer au récepteur CCR5, bloquant ainsi la pénétration du virus dans la cellule hôte . Le médicament agit comme un modulateur allostérique négatif du récepteur CCR5, induisant un changement conformationnel qui inhibe l'interaction entre le récepteur et le virus .
Analyse Biochimique
Biochemical Properties
Maraviroc selectively binds to the human chemokine receptor CCR5 present on the membrane of CD4 cells (T-cells), preventing the interaction of HIV-1 gp120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells . This interaction is crucial for the biochemical reactions involving this compound.
Cellular Effects
This compound, by binding to CCR5, blocks HIV from entering human cells . This influences cell function by preventing the virus from integrating into the host genome, thus preventing the production of new viral particles. This has a significant impact on cell signaling pathways, gene expression, and cellular metabolism.
Molecular Mechanism
This compound is an entry inhibitor and works by blocking HIV from entering human cells . Specifically, this compound is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120 . This prevents the virus from fusing with the human cell membrane .
Temporal Effects in Laboratory Settings
The effects of this compound have been studied over time in laboratory settings. This compound is extensively metabolized by CYP3A4, with renal clearance accounting for approximately 23% of total clearance . The half-life of this compound is approximately 16 hours .
Metabolic Pathways
This compound is extensively metabolized by CYP3A4 . This enzyme plays a crucial role in the metabolic pathway of this compound. The major metabolic pathways of this compound involve oxidation and N-dealkylation .
Transport and Distribution
This compound does not inhibit any of the three studied ABC transporters, and its permeability is not affected by ABCG2 or ABCC2 . This compound shows affinity for human ABCB1 and the endogenous canine Abcb1 expressed in MDCKII cells . This suggests that ABCB1/Abcb1 facilitate in situ this compound transport .
Méthodes De Préparation
Maraviroc peut être synthétisé à l'aide de diverses méthodes. Cette méthode implique l'alkylation directe d'une amine avec un alcool dans des conditions technologiquement acceptables . Le procédé comprend des étapes d'isolement et de purification améliorées pour obtenir du this compound de haute pureté . Les méthodes de production industrielle suivent généralement des voies de synthèse similaires mais sont optimisées pour une fabrication à grande échelle afin d'assurer la cohérence et l'efficacité .
Analyse Des Réactions Chimiques
Maraviroc subit plusieurs types de réactions chimiques, notamment:
Oxydation: this compound peut être oxydé dans des conditions spécifiques, conduisant à la formation de divers produits d'oxydation.
Réduction: Les réactions de réduction peuvent modifier les groupes fonctionnels dans this compound, modifiant ses propriétés chimiques.
Substitution: This compound peut subir des réactions de substitution où un groupe fonctionnel est remplacé par un autre. Les réactifs couramment utilisés dans ces réactions comprennent les agents oxydants, les agents réducteurs et divers catalyseurs.
Comparaison Avec Des Composés Similaires
Maraviroc est unique parmi les agents antirétroviraux car il cible un récepteur humain plutôt que le virus lui-même . Des composés similaires comprennent d'autres antagonistes du CCR5, tels que:
Vicriviroc: Un autre antagoniste du CCR5 avec des mécanismes d'action similaires mais des propriétés pharmacocinétiques différentes.
Propriétés
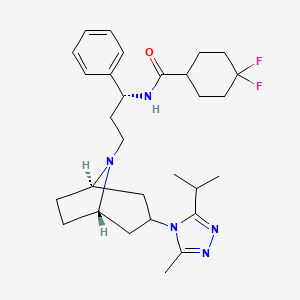
IUPAC Name |
4,4-difluoro-N-[3-[3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37) | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GSNHKUDZZFZSJB-UHFFFAOYSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NN=C(N1C2CC3CCC(C2)N3CCC(C4=CC=CC=C4)NC(=O)C5CCC(CC5)(F)F)C(C)C | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C29H41F2N5O | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
513.7 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.