马拉维罗克
描述
作用机制
科学研究应用
马拉维洛克具有广泛的科学研究应用:
生化分析
Biochemical Properties
Maraviroc selectively binds to the human chemokine receptor CCR5 present on the membrane of CD4 cells (T-cells), preventing the interaction of HIV-1 gp120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells . This interaction is crucial for the biochemical reactions involving Maraviroc.
Cellular Effects
Maraviroc, by binding to CCR5, blocks HIV from entering human cells . This influences cell function by preventing the virus from integrating into the host genome, thus preventing the production of new viral particles. This has a significant impact on cell signaling pathways, gene expression, and cellular metabolism.
Molecular Mechanism
Maraviroc is an entry inhibitor and works by blocking HIV from entering human cells . Specifically, Maraviroc is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120 . This prevents the virus from fusing with the human cell membrane .
Temporal Effects in Laboratory Settings
The effects of Maraviroc have been studied over time in laboratory settings. Maraviroc is extensively metabolized by CYP3A4, with renal clearance accounting for approximately 23% of total clearance . The half-life of Maraviroc is approximately 16 hours .
Metabolic Pathways
Maraviroc is extensively metabolized by CYP3A4 . This enzyme plays a crucial role in the metabolic pathway of Maraviroc. The major metabolic pathways of Maraviroc involve oxidation and N-dealkylation .
Transport and Distribution
Maraviroc does not inhibit any of the three studied ABC transporters, and its permeability is not affected by ABCG2 or ABCC2 . Maraviroc shows affinity for human ABCB1 and the endogenous canine Abcb1 expressed in MDCKII cells . This suggests that ABCB1/Abcb1 facilitate in situ Maraviroc transport .
准备方法
马拉维洛克可以使用多种方法合成。 这种方法涉及在技术上可接受的条件下,胺与醇的直接烷基化 . 该工艺包括改进的隔离和纯化步骤,以获得高纯度的马拉维洛克 . 工业生产方法通常遵循类似的合成路线,但针对大规模生产进行了优化,以确保一致性和效率 .
化学反应分析
马拉维洛克经历了几种类型的化学反应,包括:
氧化: 马拉维洛克可以在特定条件下被氧化,导致形成各种氧化产物。
还原: 还原反应可以改变马拉维洛克中的官能团,改变其化学性质。
取代: 马拉维洛克可以发生取代反应,其中一个官能团被另一个官能团取代。这些反应中常用的试剂包括氧化剂、还原剂和各种催化剂.
相似化合物的比较
马拉维洛克在抗逆转录病毒药物中是独一无二的,因为它靶向人类受体,而不是病毒本身 . 类似的化合物包括其他 CCR5 拮抗剂,例如:
维克瑞维洛克: 另一种 CCR5 拮抗剂,具有类似的作用机制,但药代动力学性质不同。
阿普拉维洛克: 一种 CCR5 拮抗剂,由于安全问题而停产。马拉维洛克的独特之处在于其良好的安全特性以及能够与其他抗逆转录病毒药物联合使用而不会产生明显药物相互作用.
属性
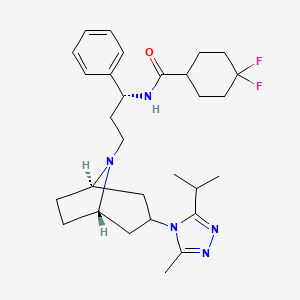
IUPAC Name |
4,4-difluoro-N-[3-[3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37) | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GSNHKUDZZFZSJB-UHFFFAOYSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NN=C(N1C2CC3CCC(C2)N3CCC(C4=CC=CC=C4)NC(=O)C5CCC(CC5)(F)F)C(C)C | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C29H41F2N5O | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
513.7 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。