Abacavir
Overview
Description
Abacavir is a synthetic carbocyclic nucleoside analog reverse transcriptase inhibitor used primarily in the treatment of Human Immunodeficiency Virus (HIV) and Acquired Immunodeficiency Syndrome (AIDS). It is marketed under the brand name Ziagen among others. This compound is typically used in combination with other antiretroviral medications and is not recommended for use alone . It is known for its ability to inhibit the replication of HIV by targeting the reverse transcriptase enzyme, which is crucial for the virus’s replication process .
Mechanism of Action
Target of Action
Abacavir is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1) . The primary target of this compound is the HIV-1 reverse transcriptase enzyme . This enzyme is crucial for the replication of the HIV virus, as it is responsible for the transcription of viral RNA into DNA .
Mode of Action
This compound is converted by cellular enzymes to the active metabolite carbovir triphosphate, an analogue of deoxyguanosine-5’-triphosphate (dGTP) . This active metabolite competes with natural nucleotides for incorporation into the viral DNA . When incorporated, it inhibits the HIV reverse transcriptase enzyme competitively and acts as a chain terminator of DNA synthesis . This prevents the virus from replicating and reduces the viral load in the body .
Biochemical Pathways
This compound interferes with the reverse transcription process, a critical step in the HIV life cycle . By inhibiting the reverse transcriptase enzyme, it prevents the conversion of viral RNA into DNA, thus blocking the integration of viral DNA into the host genome . This ultimately leads to a decrease in viral replication and an increase in CD4 cell counts .
Pharmacokinetics
This compound is rapidly absorbed following oral administration, with a mean absolute bioavailability of approximately 83% . Maximum this compound concentrations are observed in serum within 0.5 to 3.0 hours of dosing in the fasted state (median Tmax of 1 - 1.5 hour) . The elimination half-life of this compound is 1.5 hours . It is primarily metabolized through cytosolic alcohol dehydrogenase (ADH) and uridine diphosphate glucuronosyltransferase (UGT) enzymes .
Action Environment
The efficacy and stability of this compound can be influenced by various environmental factors. For instance, genetic factors can play a significant role. The presence of the HLA-B*57:01 allele is strongly linked to the occurrence of a hypersensitivity reaction to this compound . Therefore, testing for this allele prior to this compound treatment is recommended . Additionally, drug interactions can also influence the action of this compound. Since this compound is primarily metabolized by ADH and UGT enzymes, no interactions between this compound and inducers or inhibitors of cytochrome P450 (CYP) enzymes are predicted .
Preparation Methods
Synthetic Routes and Reaction Conditions: The synthesis of abacavir involves several steps, starting from a suitable di-halo aminopyrimidine compound. The key steps include:
Reaction with Aminoalcohol: The di-halo aminopyrimidine is reacted with an aminoalcohol to form an intermediate compound.
Cyclization: This intermediate undergoes cyclization to form a key intermediate.
Introduction of Cyclopropylamine: The chlorine atom in the intermediate is displaced with cyclopropylamine to yield this compound as a free base.
Industrial Production Methods: Industrial production of this compound often involves optimizing the reaction conditions to ensure high yield and purity. This includes controlling the temperature, pH, and the use of specific catalysts to facilitate the reactions. The process may also involve purification steps such as crystallization and chromatography to isolate the final product .
Types of Reactions:
Oxidation: this compound undergoes oxidative degradation, which can be studied using electrochemical methods.
Reduction: While specific reduction reactions of this compound are less documented, it is possible that under certain conditions, this compound could undergo reduction.
Substitution: The synthesis of this compound itself involves substitution reactions, particularly the displacement of chlorine with cyclopropylamine.
Common Reagents and Conditions:
Oxidation: Hydrogen peroxide, platinum electrodes, boron-doped diamond electrodes.
Substitution: Cyclopropylamine, various solvents and catalysts.
Major Products:
Scientific Research Applications
Abacavir has a wide range of applications in scientific research, particularly in the fields of chemistry, biology, and medicine:
Chemistry: this compound serves as a model compound for studying nucleoside analogs and their interactions with enzymes.
Biology: It is used to study the mechanisms of viral replication and the development of resistance in HIV.
Medicine: this compound is a critical component of antiretroviral therapy for HIV/AIDS.
Comparison with Similar Compounds
Lamivudine: Another nucleoside reverse transcriptase inhibitor used in combination with abacavir.
Zidovudine: An older nucleoside reverse transcriptase inhibitor with a similar mechanism of action.
Tenofovir: A nucleotide reverse transcriptase inhibitor with a different chemical structure but similar antiviral activity.
Comparison:
Uniqueness: this compound is unique in its structure as a carbocyclic nucleoside analog. It has a distinct cyclopropylamine group that differentiates it from other nucleoside analogs.
Safety Profile: this compound has a well-documented safety profile, though it is associated with a risk of hypersensitivity reactions and potential cardiovascular risks.
Properties
IUPAC Name |
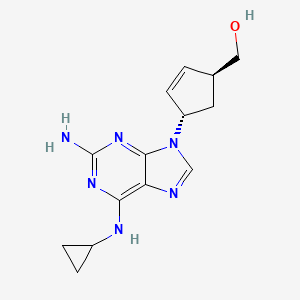
[4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl]methanol | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H18N6O/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19) | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
MCGSCOLBFJQGHM-UHFFFAOYSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC1NC2=C3C(=NC(=N2)N)N(C=N3)C4CC(C=C4)CO | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H18N6O | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID20861337 | |
| Record name | 4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20861337 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
286.33 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
136470-78-5, 914348-29-1 | |
| Record name | NSC742406 | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=742406 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID20861337 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.


![ethyl (2S)-2-[[2-(acetylsulfanylmethyl)-3-(2-methylphenyl)propanoyl]amino]-4-methylsulfanylbutanoate](/img/structure/B1662769.png)
![1-(7-(4-Fluorophenyl)-8-(pyridin-4-yl)-3,4-dihydropyrazolo[5,1-c][1,2,4]triazin-2(1H)-yl)-2-phenylethane-1,2-dione sulfate](/img/structure/B1662783.png)