Maraviroc
Overview
Description
Maraviroc is a chemokine receptor antagonist developed by Pfizer, marketed under the brand names Selzentry in the United States and Celsentri in the European Union . It is primarily used as an antiretroviral medication for the treatment of CCR5-tropic HIV-1 infection . This compound works by blocking the CCR5 receptor on the surface of certain human cells, preventing the HIV virus from entering these cells .
Mechanism of Action
Target of Action
Maraviroc, developed by Pfizer, is a chemokine receptor antagonist drug . Its primary target is the human chemokine receptor CCR5 . This receptor is present on the membrane of CD4 cells (T-cells), which are a type of white blood cell that plays a key role in the immune system .
Mode of Action
This compound works by blocking HIV from entering human cells . Specifically, it is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120 . By binding to CCR5, this compound prevents the interaction of HIV-1 gp120 with CCR5-tropic HIV-1, thereby inhibiting the virus from entering the cell .
Biochemical Pathways
This compound’s action affects the HIV entry process . The drug interferes with the interaction between the HIV-1 gp120 protein and the CCR5 receptor, which is an essential step for the virus to enter the host cell . By blocking this interaction, this compound prevents the virus from fusing with the human cell membrane .
Pharmacokinetics
This compound is extensively metabolized by CYP3A4 , with renal clearance accounting for approximately 23% of total clearance . The half-life of this compound is approximately 16 hours .
Result of Action
The molecular and cellular effects of this compound’s action result in the inhibition of HIV-1 entry into human cells . By preventing the virus from entering the cells, this compound can effectively reduce the progression of HIV-1 infection .
Action Environment
The efficacy and stability of this compound can be influenced by various environmental factors. For instance, the sensitivity of tropism testing can impact the treatment outcome of this compound-containing regimens . Additionally, this compound’s exposure is altered by agents that modulate the activity of CYP3A4, and in some circumstances, this compound dose adjustment is necessary .
Biochemical Analysis
Biochemical Properties
Maraviroc selectively binds to the human chemokine receptor CCR5 present on the membrane of CD4 cells (T-cells), preventing the interaction of HIV-1 gp120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells . This interaction is crucial for the biochemical reactions involving this compound.
Cellular Effects
This compound, by binding to CCR5, blocks HIV from entering human cells . This influences cell function by preventing the virus from integrating into the host genome, thus preventing the production of new viral particles. This has a significant impact on cell signaling pathways, gene expression, and cellular metabolism.
Molecular Mechanism
This compound is an entry inhibitor and works by blocking HIV from entering human cells . Specifically, this compound is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120 . This prevents the virus from fusing with the human cell membrane .
Temporal Effects in Laboratory Settings
The effects of this compound have been studied over time in laboratory settings. This compound is extensively metabolized by CYP3A4, with renal clearance accounting for approximately 23% of total clearance . The half-life of this compound is approximately 16 hours .
Metabolic Pathways
This compound is extensively metabolized by CYP3A4 . This enzyme plays a crucial role in the metabolic pathway of this compound. The major metabolic pathways of this compound involve oxidation and N-dealkylation .
Transport and Distribution
This compound does not inhibit any of the three studied ABC transporters, and its permeability is not affected by ABCG2 or ABCC2 . This compound shows affinity for human ABCB1 and the endogenous canine Abcb1 expressed in MDCKII cells . This suggests that ABCB1/Abcb1 facilitate in situ this compound transport .
Preparation Methods
Maraviroc can be synthesized using various methods. This method involves the direct alkylation of an amine with an alcohol under technologically acceptable conditions . The process includes improved isolation and purification steps to obtain high-purity this compound . Industrial production methods typically follow similar synthetic routes but are optimized for large-scale manufacturing to ensure consistency and efficiency .
Chemical Reactions Analysis
Maraviroc undergoes several types of chemical reactions, including:
Oxidation: this compound can be oxidized under specific conditions, leading to the formation of various oxidation products.
Reduction: Reduction reactions can modify the functional groups within this compound, altering its chemical properties.
Substitution: This compound can undergo substitution reactions where one functional group is replaced by another. Common reagents used in these reactions include oxidizing agents, reducing agents, and various catalysts.
Scientific Research Applications
Maraviroc has a wide range of scientific research applications:
Comparison with Similar Compounds
Maraviroc is unique among antiretroviral agents as it targets a human receptor rather than the virus itself . Similar compounds include other CCR5 antagonists, such as:
Vicriviroc: Another CCR5 antagonist with similar mechanisms of action but different pharmacokinetic properties.
Aplaviroc: A CCR5 antagonist that was discontinued due to safety concerns. This compound’s uniqueness lies in its favorable safety profile and its ability to be used in combination with other antiretroviral agents without significant drug interactions.
Properties
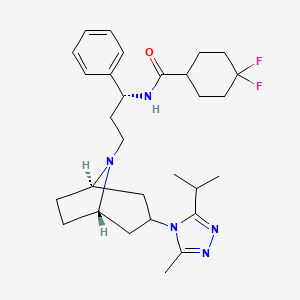
IUPAC Name |
4,4-difluoro-N-[3-[3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide | |
|---|---|---|
| Details | Computed by Lexichem TK 2.7.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37) | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GSNHKUDZZFZSJB-UHFFFAOYSA-N | |
| Details | Computed by InChI 1.0.6 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=NN=C(N1C2CC3CCC(C2)N3CCC(C4=CC=CC=C4)NC(=O)C5CCC(CC5)(F)F)C(C)C | |
| Details | Computed by OEChem 2.3.0 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C29H41F2N5O | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
513.7 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.